Keep up to date with the latest research and developments from Diamond. Sign up for news on our scientific output, facility updates and plans for the future.
Related publication: Beale A. M., Greenaway A. G., Kroner A. B., Lezcano-González I., Agote-Arán M., Hayama S. & Díaz-Moreno S. Operando HERFD-XANES/XES studies reveal differences in the activity of Fe-species in MFI and CHA structures for the standard selective catalytic reduction of NO with NH3. Appl. Catal. A Gen. 570, 283–291 (2018). DOI: 10.1016/j.apcata.2018.11.026
Diesel is the fuel of choice for heavy goods vehicles, but diesel combustion generates nitrogen oxides (NOx) that are known to be harmful to human health; NOx has been estimated to lead to 38,000 premature deaths globally each year. Selective catalytic reduction with ammonia (NH3) is a widely-applied technology for converting NOx emissions from diesel engines into harmless nitrogen gas and water.
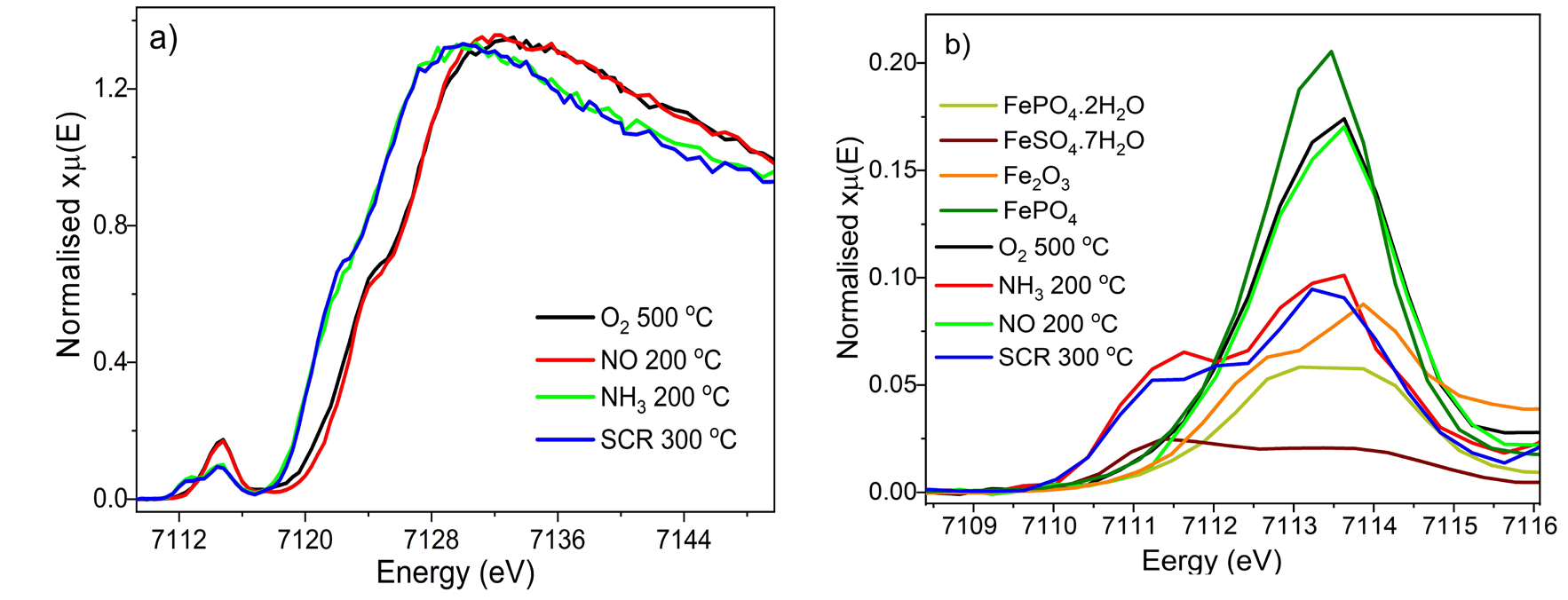
The HERFD-XANES data reveal a dynamic chemical state of Fe in Fe/H-ZSM-5 that changes with gas atmosphere. The pre-edge features of the catalyst before reaction suggests the presence of mainly isolated Fe3+ species with both Oh and Td species. Small changes in the XANES spectrum can be observed when flowing NO, which has previously been attributed to NO adsorption onto Fe3+ centres, leading to a partial Fe reduction (i.e. oxidative addition of NO). In the presence of reductive gases, however, bigger changes are observed; the pre-edge is seen to shift to lower energies under NH3 (centroid position goes from 7113.41 to 7112.95 eV), indicating reduction to Fe2+ - probably due to ammonia coordination to the metal, and donation of the free electron pair of the nitrogen, resulting in the formation of Fe2+-NH2 complexes. A similar shift is observed under NH3-SCR conditions, and is consistent with reoxidation of Fe2+ to Fe3+ being a slow step in the reaction process. Note that during reaction, a mass spectrometer was used to verify that the catalyst was actively reducing NOx.
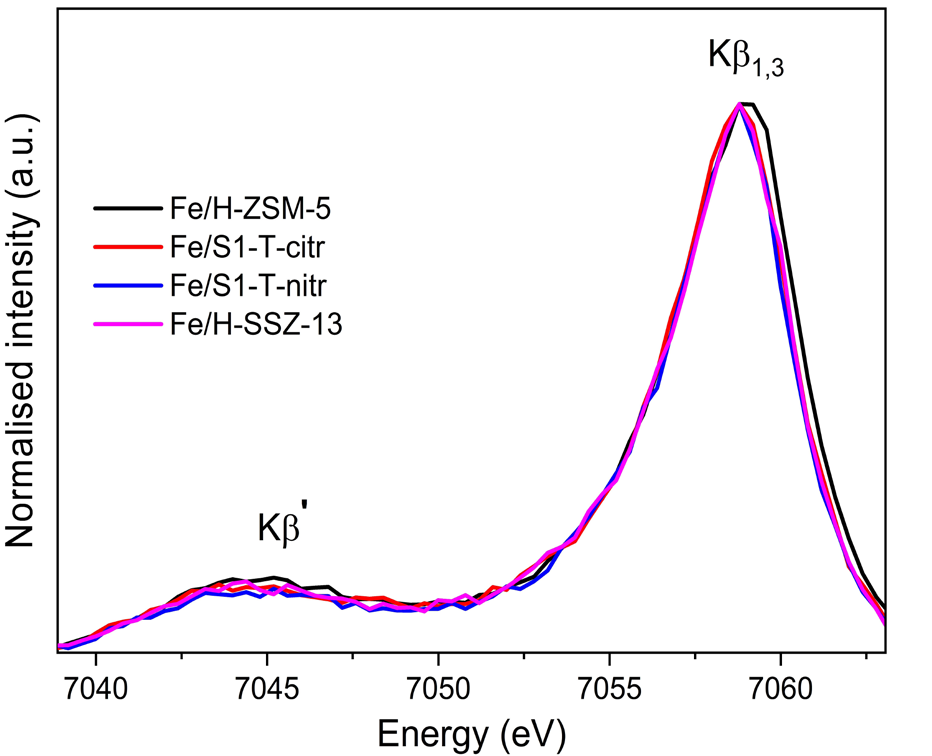
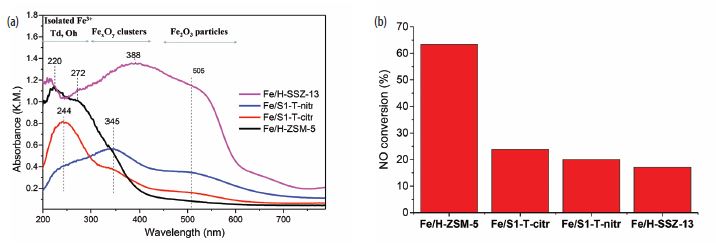
In addition to the HERFD-XANES data discussed above, Kβ XES spectra were also acquired. Fig. 3 shows the Kβ’ and Kβ1,3 mainlines (3p→1s transitions of the absorbing atom) for the Fe/zeolites (recorded at room temperature after calcination). All the spectra present a well-defined Kβ’ feature, indicating they constitute high-spin complexes. For metal complexes with the same spin-state, the centroid of the Kβ1,3 feature can be correlated with the covalent (vs. ionic) character of the metal-ligand bond; it has been reported that Kβ1,3 emission shifts to higher energies with increasing ionic character. In Fig. 3, it appears that all the spectra seem identical although the Kβ1,3 peak in the Fe/H-ZSM-5 sample is slightly shifted to higher energies (i.e. Kβ1,3 maxima at 7059.15 eV while for the rest of the references is at 7058.78 eV). Such a shift may be indicative of a different, more ionic, metal-ligand bond character with respect to the other samples. This could be a consequence of the fact that Fe is providing charge compensation of the framework AlO4- charge. This is not the case of Fe/ Silicalite-1 catalysts as these materials do not contain framework Al, while for Fe/H-SSZ-13 the majority of the species present are Fe2O3 particles.
Diamond Light Source is the UK's national synchrotron science facility, located at the Harwell Science and Innovation Campus in Oxfordshire.
Copyright © 2022 Diamond Light Source
Diamond Light Source Ltd
Diamond House
Harwell Science & Innovation Campus
Didcot
Oxfordshire
OX11 0DE
Diamond Light Source® and the Diamond logo are registered trademarks of Diamond Light Source Ltd
Registered in England and Wales at Diamond House, Harwell Science and Innovation Campus, Didcot, Oxfordshire, OX11 0DE, United Kingdom. Company number: 4375679. VAT number: 287 461 957. Economic Operators Registration and Identification (EORI) number: GB287461957003.