Keep up to date with the latest research and developments from Diamond. Sign up for news on our scientific output, facility updates and plans for the future.
Over the past few years, responsive materials - materials that change their structure when chemical, thermal, or mechanical stimuli are applied - have been studied extensively. A team of researchers used high-pressure X-ray diffraction on the Extreme Conditions beamline (I15) to study stimuli-responsive metal-organic frameworks (MOFs), a class of synthetic, crystalline, and porous materials constructed from organic and inorganic building units. Some MOFs are known to be flexible, and to dramatically change their crystal structure upon adsorption of guest molecules or changes in temperature. The team wanted to investigate whether the responsive behaviour of MOFs can also be triggered by mechanical compression.
They focused on ZIF-4 materials, which are representative of an important subclass of MOFs called zeolitic imidazolate frameworks (ZIFs). They had previously shown that the Zn-based derivative ZIF-4(Zn) undergoes a remarkable phase transition from an open-pore form to a closed-pore form when cooled to cryogenic temperatures, and now wanted to investigate whether the same change could also be triggered by compressing the material. If so, the materials would have potential for shock absorbers, nanodampers, and cooling applications.
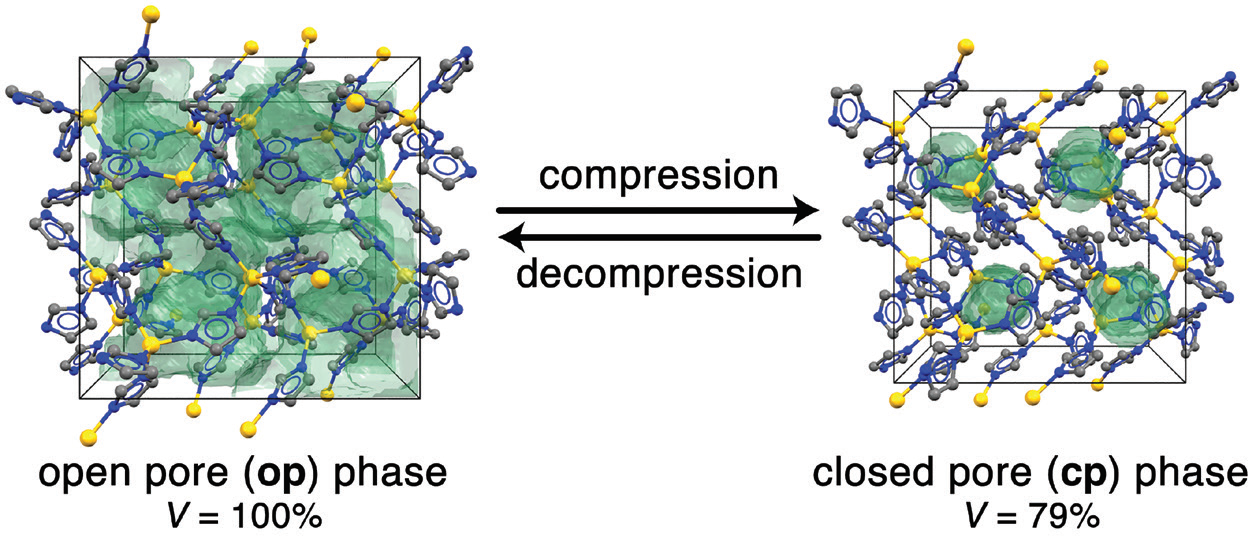
Metal-organic frameworks (MOFs) are a class of crystalline porous materials constructed from molecular organic and inorganic building units. Some MOFs feature remarkable responsiveness towards external stimuli, enabling them to undergo reversible phase transitions as a function of guest molecule adsorption or changes in temperature1,2. Importantly, the crystalline order and internal connectivity (also known as topology) of the frameworks is retained in these processes, while volumetric changes of more than 20% occur. Such transitions typically involve large changes in pore volume, pore size, and pore shape. Chemical bonds between the building units remain fully intact, while bond and torsional angles change. In contrast to guest- and temperature-dependent structural changes, phase transitions as a function of mechanical pressure are much less common for MOFs3,4,5. However, pressure-driven framework flexibility is appealing for the application of such framework materials as shock absorbers, nanodampers, or for cooling applications (i.e. mechanocalorics).
Herein, the high-pressure phase behaviour of two isostructural zeolitic imidazolate frameworks (ZIFs) named ZIF-4(M), featuring the chemical composition M(im)2 (with M2+ = Zn2+ or Co2+, im– = imidazolate), was studied. ZIF-4(M) crystallises in the orthorhombic space group Pbca and features the cag topology. During a previous beamtime at the High Resolution Powder Diffraction beamline (I11) at Diamond, the team discovered that ZIF-4(Zn) undergoes a remarkable phase transition from its conventional open-pore (op) phase to a closed-pore (cp) phase when cooled below 140 K (Fig. 1)2. The cp phase is about 30% denser than the porous op phase; space group symmetry and network topology, however, are unchanged. During the transition a concerted sequence of single-bond rotations results in an isotropic contraction of the framework by more than 20% in volume.
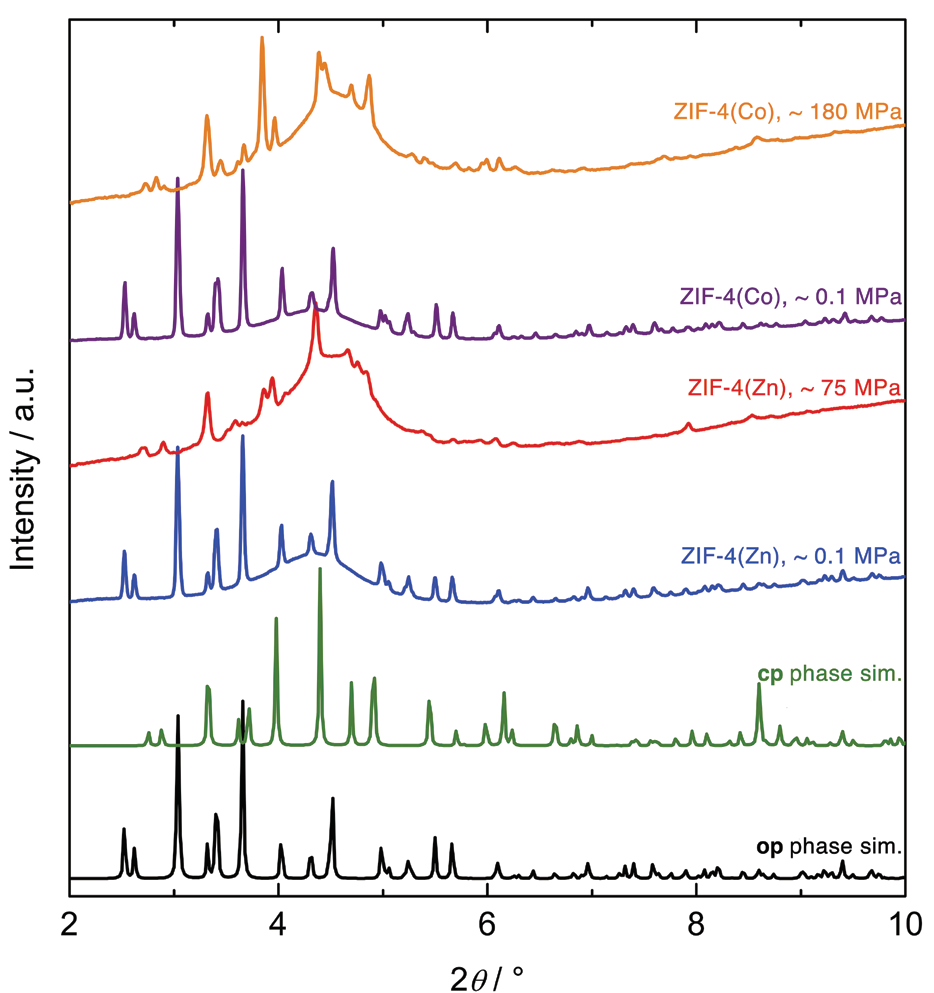
In the current study, High-Pressure Powder X-ray Diffraction (HP-PXRD) experiments were performed at beamline I15 at Diamond to investigate if ZIF-4(Zn), and its isostructural derivative ZIF-4(Co), undergo similar op-cp transitions when exposed to hydrostatic mechanical pressures. Finely ground and guest-free powders of the ZIF-4(M) materials were loaded into membrane diamond anvil cells (mDAC), together with a pressure transmitting fluid (PTF), and an alkali halide, as internal pressure standard. mDAC preparation had to be performed inside a glovebox (Ar atmosphere) in order to prevent adsorption of moisture inside the guest-free porous ZIF-4(M) compounds. Fluorinert FC-70, a liquid composed of molecules that are too large to penetrate into the microporous frameworks of the ZIF-4(M) compounds, was used as PTF. Subsequently, HP-PXRD patterns were recorded as a function of hydrostatic pressure. Surprisingly, the op-cp phase transitions of the ZIF-4(M) compounds were observed already at comparatively low hydrostatic pressures between 75 and 180 MPa (Fig. 2). Structureless profile fitting suggested that the high-pressure cp phases of ZIF-4(Zn) and ZIF-4(Co) are similar, but different to the previously reported low temperature cp phase of ZIF-4(Zn). The HP-PXRD patterns could not be fitted satisfactory in the expected orthorhombic symmetry (space group Pbca). However, a symmetry reduction involving a small shear element, and a transition to the monoclinic subgroup P21/c, yielded a reasonable fit to both experimental datasets. A volumetric compression by ~21% was found for ZIF-4(Zn), and by ~19% for ZIF-4(Co).
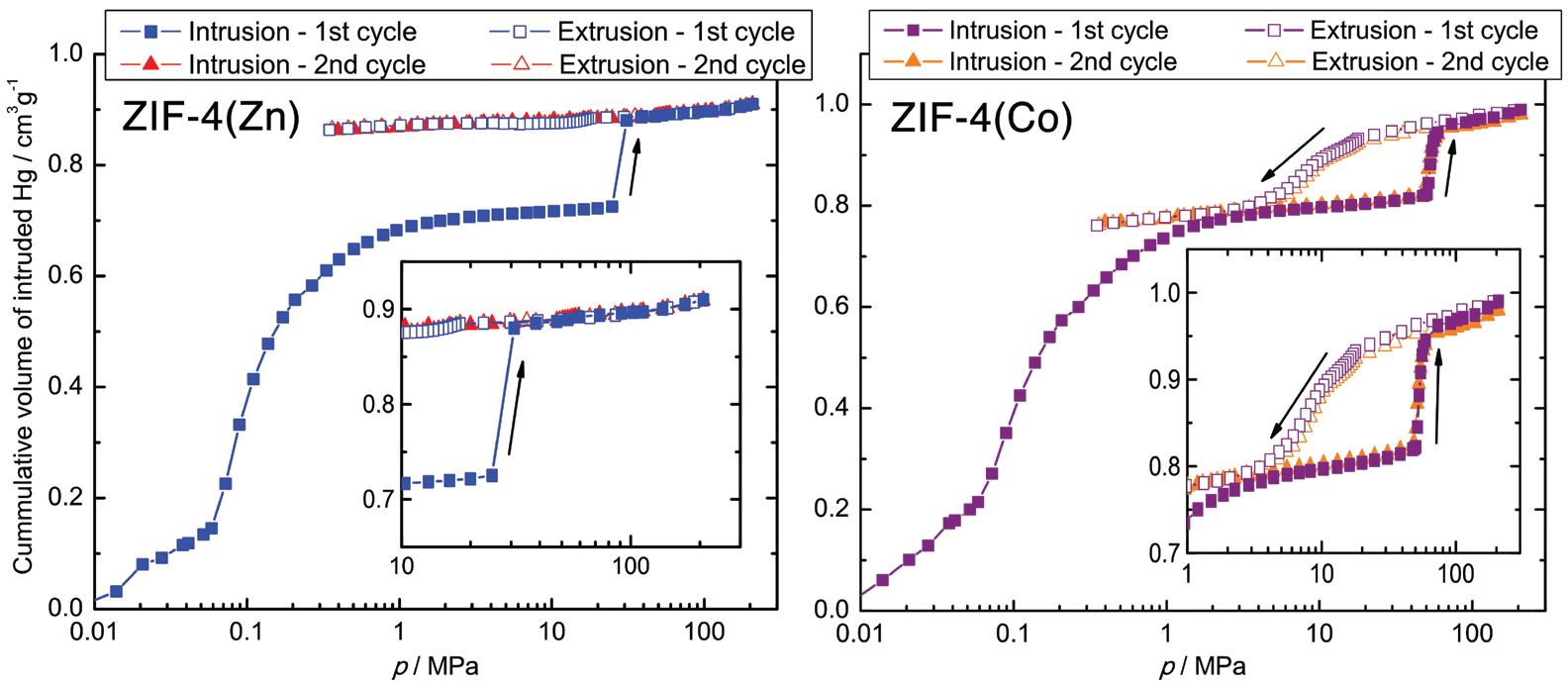
ZIF-4(Co) shows a different behaviour. The step associated to the op-cp phase transition is present at a much higher pressure of ~50 MPa, and the reverse cp-op transition is visible as a shallow but distinct step at pressures below 20 MPa. A second intrusion-extrusion cycle underlines the full reversibility of the phase transition process for ZIF-4(Co). The apparent differences of ZIF-4(Zn) and ZIF-4(Co) can be attributed to the specific valence electron configurations and electronegativities (ENs) of their respective metal ions (Zn2+, 3d10, EN = 1.65; Co2+, 3d7, EN = 1.88), which suggest a stronger and more directional ligand to metal bonding, as well as stiffer coordination tetrahedra for the Co-based derivative.
References:
Diamond Light Source is the UK's national synchrotron science facility, located at the Harwell Science and Innovation Campus in Oxfordshire.
Copyright © 2022 Diamond Light Source
Diamond Light Source Ltd
Diamond House
Harwell Science & Innovation Campus
Didcot
Oxfordshire
OX11 0DE
Diamond Light Source® and the Diamond logo are registered trademarks of Diamond Light Source Ltd
Registered in England and Wales at Diamond House, Harwell Science and Innovation Campus, Didcot, Oxfordshire, OX11 0DE, United Kingdom. Company number: 4375679. VAT number: 287 461 957. Economic Operators Registration and Identification (EORI) number: GB287461957003.