Keep up to date with the latest research and developments from Diamond. Sign up for news on our scientific output, facility updates and plans for the future.
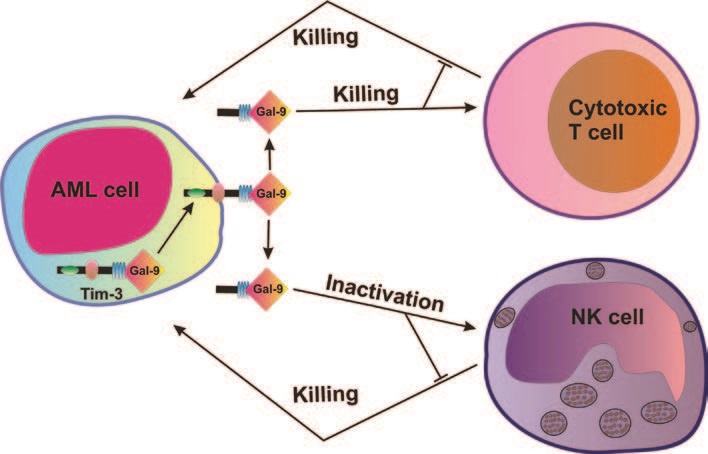
Acute myeloid leukemia (AML) is an aggressive blood/bone marrow cancer originating from self-renewing malignant immature myeloid cells. AML rapidly becomes a systemic malignancy and is often a fatal disease since cancerous cells are suppressing anti-cancer immunity by impairing the functional activity of natural killer (NK) cells and T cells with cytotoxic activity1.
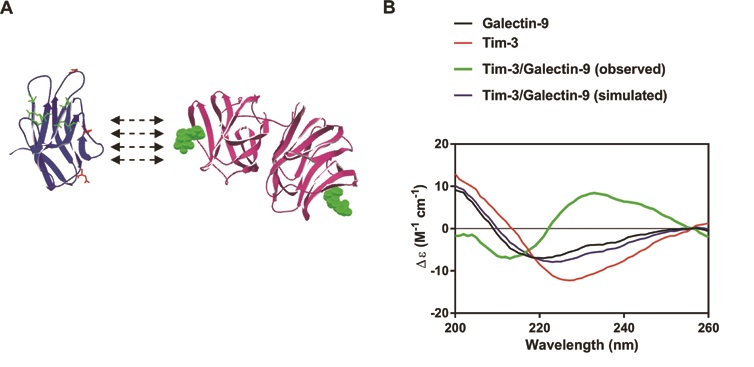
Synchrotron radiation circular dichroism (SRCD) spectroscopy at beamline B23 was used to characterise the secondary structure of Tim-3, galectin-9 and the complex made by these two proteins presented in Figure 2a. Structural organisation of both Tim-3 and galectin-9 are presented in the Figure 2a. It was found in earlier studies that galectin-9 interacts with non-glycosylated Tim-3 with high affinity (Kd = 2.8 × 10−8 M)4. However, this binding is further strengthened by interaction of galectin-9 with sugars of glycosylated Tim-3. In AML cells Tim-3 undergoes glycosylation2 which means that its affinity to galectin-9 is very high in AML cells. This assumption was confirmed by the fact that the complex is detectable by Western blot analysis, which suggests a strong high affinity binding between the two proteins2,5. SRCD spectroscopy was also applied to galectin-9 and Tim-3 mixed to a stoichiometry of 1:1 molar ratio (Fig. 2b). When mixed together with Tim-3, galectin-9 showed a CD spectrum which was clearly different from the simulated spectrum. This suggested that the interaction of Tim-3 with galectin-9 caused a conformational change of both proteins. An obvious increase in β-strand component was observed. Based on these findings, one may suggest that Tim-3 binding possibly alters the conformation of galectin-9. This results in increased capability of galectin-9 to interact with receptors in target cells. Galectin-9 is a protein, which contains two sugar-binding domains. Therefore, one domain could bind Tim-3 (or other proteins/receptor/trafficker) and leave the other domain open for interaction with a receptor molecule (for example Tim-3) associated with the plasma membrane of a target cell (for example NK cell or cytotoxic T cell).
Gonçalves Silva I, Yasinska IM, Sakhnevych SS, Fiedler W, Wellbrock J, Bardelli M, Varani L, Hussain R, Siligardi G, Ceccone G, Berger SM, Ushkaryov YA, Gibbs BF, Fasler-Kan E, Sumbayev VV. The Tim-3-galectin-9 Secretory Pathway is Involved in the Immune Escape of Human Acute Myeloid Leukemia Cells. EBioMedicine 22, 44 – 57, doi:10.1016/j.ebiom.2017.07.018 (2017).
Diamond Light Source is the UK's national synchrotron science facility, located at the Harwell Science and Innovation Campus in Oxfordshire.
Copyright © 2022 Diamond Light Source
Diamond Light Source Ltd
Diamond House
Harwell Science & Innovation Campus
Didcot
Oxfordshire
OX11 0DE
Diamond Light Source® and the Diamond logo are registered trademarks of Diamond Light Source Ltd
Registered in England and Wales at Diamond House, Harwell Science and Innovation Campus, Didcot, Oxfordshire, OX11 0DE, United Kingdom. Company number: 4375679. VAT number: 287 461 957. Economic Operators Registration and Identification (EORI) number: GB287461957003.