NiO and MgO crystals appear transparent to the human eye as they do not absorb light in the visible region of the electromagnetic spectrum, instead only absorbing the high-energy ultraviolet (UV) radiation. While NiO readily absorbs long-wavelength UV light at the edge of the visible spectrum, MgO is even more transparent than ozone for short-wavelength UV radiation. Thanks to this complementary absorption behaviour, crystals made from solid solutions of both NiO and MgO are promising materials for wavelengthtunable UV light detectors. However, when crystals of NixMg1-xO crystals are produced their absorption properties do not follow that expected from a homogeneous mixture of the two components.
To discover the reason behind the anomaly, researchers turned to the Test beamline (B16) at Diamond Light Source. They carried out X-ray diffraction experiments to determine the dimension of the smallest periodic structural unit of NixMg1-xO crystals, the so-called lattice parameter. Due to the near-identical lattice parameters of NiO and MgO it is extremely difficult to clearly resolve the diffraction signals of NixMg1-xO crystals with varying composition x. However, the low-energy synchrotron radiation provided by B16 allowed the researchers to distinguish the signals with significantly enhanced resolution.
Researchers previously assumed that the anomalous behaviour was due to a compositional inhomogeneity of the NixMg1-xO crystals. However, the high-resolution diffraction experiments at Diamond showed that NiO and MgO formed fully miscible crystals, effectively disproving this theory. Instead the researchers’ computer-aided theoretical calculations showed that the origin of this behaviour lies in an anomaly occurring in the electronic structure of the material, which had been overlooked in previous studies attempting to unravel the intriguing UV light absorption of NixMg1-xO crystals.
The wide-band gap semiconductor NiO (3.7 eV) is one of the rare p-type transparent conductive oxides demonstrating good electrical properties for application in optoelectronic devices. NiO forms a fully miscible solid solution with MgO (7.8 eV), offering a great flexibility for band gap tuning in the deep UV spectral region without the drawbacks of a significant change in lattice parameter or a phase transition. Therefore, the NixMg1-xO solid solution is increasingly receiving interest for application in deep UV wavelength-tuneable photodetectors.
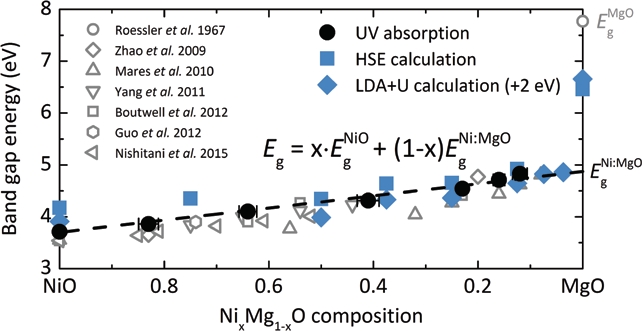
To adjust the photosensitivity of NixMg1-xO UV detectors, it is essential to understand the optical absorption as a function of composition x. Experimentally it has been shown that the band gap of semiconductor alloys is well described by a linear interpolation of the band gaps of its components, and taking into account the parabolic band gap narrowing proportional to x(1-x) [1], referred to as ‘band gap bowing’. Even though NiO and MgO both crystallise in the cubic rock-salt structure with very close lattice parameters of less than 0.8% relative difference, the band gap of the solid solution strongly deviates from the standard bowing behaviour [2], which has led to controversial discussions in the literature. Regardless of the clearly non-parabolic band gap dependence on composition, the parabolic bowing equation has been rigidly applied in many previous studies to describe the band gap evolution in the NixMg1-xO system.
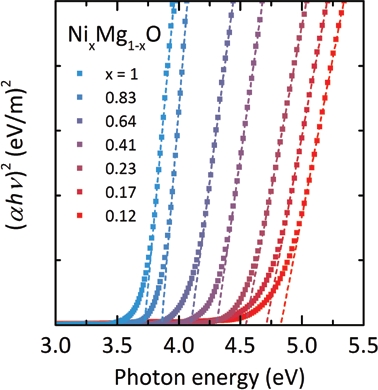
The present work determined the photon energy at the onset of UV absorption of visible-transparent NixMg1-xO epitaxial thin films prepared by pulsed laser deposition on MgO substrates. The optical absorption spectra confirm the linear relationship between the photon energy hν and (αhν)2, where α denotes the absorption coefficient, which is valid for direct transitions in semiconductors at the absorption edge (Fig. 1). The NixMg1-xO optical band gap energy as determined from the absorption spectra shows a linear dependence over the entire investigated composition range (1 ≤ x ≤ 0.12) from 3.7 to 4.8 eV (Fig. 2). Despite the small NiO fraction in Ni0.12Mg0.88O, it is striking to observe that the band gap is smaller by 3 eV as compared to MgO.
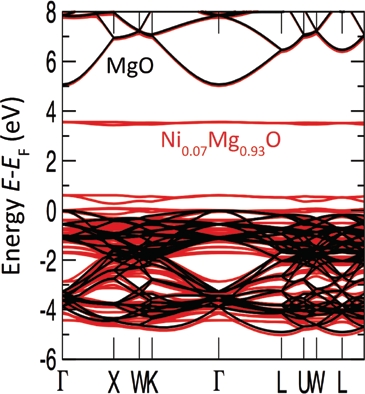
A superposition of the calculated band structures of Ni0.07Mg0.93O and MgO shows deep localised levels almost entirely derived from Ni 3d eg states at about 3 eV within the MgO band gap (Fig. 3). Both the Ni0.07Mg0.93O valence and conduction band show an otherwise unperturbed band structure derived from MgO. The Ni 3d eg impurity-like level is strongly localised due to the negligible contribution from hybridisation with O 2p states of the surrounding atoms and has been predicted in Ni-doped MgO [3]. As has been investigated for the GaAsxN1-x alloy, such localised levels in the dilute composition domain (x → 0) evoke largely irregular electronic properties [4].
The NixMg1-xO solid solution presents a unique system in which cation substitution of a small percentage of Ni2+ for Mg2+ leads to a fundamental change in the electronic structure, while maintaining complete miscibility as well as structural stability over the entire composition range. The composition dependence of the NixMg1-xO band gap is best described by a linear interpolation of the NiO band gap Eg NiO (3.7 eV) and that of MgO doped with an infinitesimal Ni impurity concentration Eg Ni:MgO (4.9 eV).
In conclusion, the standard bowing theory is inapplicable to describe the non-parabolic composition dependence of the NixMg1-xO band gap, because deep localised Ni 3d gap states evoke a largely irregular band gap narrowing in the dilute NiO concentration domain (0.13 ≤ x ≤ 0.04).
References:
Funding acknowledgement:
This work was supported by the Leverhulme Trust via M. A. Moram’s Research Leadership Award (RL-0072012) and the by the Royal Society through M. A. Moram’s University Research Fellowship. We thank Diamond Light Source (UK) for synchrotron experiment time on Beamline B16 (MT9149). We acknowledge use of the Imperial College high performance computing service and, via our membership of the UK’s HEC Materials Chemistry Consortium funded by EPSRC (EP/L000202), we also acknowledge the use of the ARCHER UK national supercomputing service (http://www.archer.ac.uk).
Corresponding author: Christian Niedermeier, Imperial College London, [email protected]
Related publication:
Niedermeier CA, Råsander M, Rhode S, Kachkanov V, Zou B, Alford N, Moram, MA. Band gap bowing in NixMg1-xO. Scientific Reports 6, 31230, doi:10.1038/srep31230 (2016).
Publication keywords:
Electronic structure; Optical absorption; NixMg1-xO solid solution
Diamond Light Source is the UK's national synchrotron science facility, located at the Harwell Science and Innovation Campus in Oxfordshire.
Copyright © 2022 Diamond Light Source
Diamond Light Source Ltd
Diamond House
Harwell Science & Innovation Campus
Didcot
Oxfordshire
OX11 0DE
Diamond Light Source® and the Diamond logo are registered trademarks of Diamond Light Source Ltd
Registered in England and Wales at Diamond House, Harwell Science and Innovation Campus, Didcot, Oxfordshire, OX11 0DE, United Kingdom. Company number: 4375679. VAT number: 287 461 957. Economic Operators Registration and Identification (EORI) number: GB287461957003.