THz Coherent Synchrotron Radiation for probing water-protein interaction
The latest published paper using data from the Multimode InfraRed Imaging and Microspectroscopy (MIRIAM) beamline (B22) at Diamond Light Source has shown how far InfraRed (IR) radiation can be used to understand water interaction with protein systems at the molecular level.
Although water may seem extremely commonplace, there remain a large number of unknown details as to how it interacts within biological systems. One such example is to what extent are water molecules surrounding a protein molecule in solution different to bulk water? Protein hydration has biochemical relevance, as the H2O molecules interact with the protein conformation and contribute to its multifunctional role and dynamics.
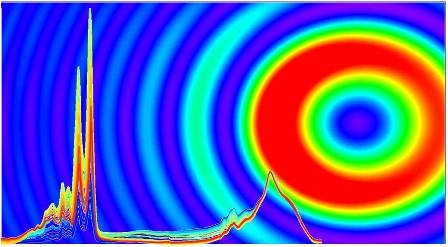
The MIRIAM beamline routinely operates for infrared microspectroscopy in the mid-IR or fingerprint region, i.e. where a one-to-one molecular-bond to IR-band correspondence applies. Recently, however, it has pushed its capabilities beyond the millimetre wavelength limit: this is commonly referred to as THz spectroscopy. “The term ‘THz gap’ denotes the difficulty of both generating and exploiting scientifically the electromagnetic radiation between what is commonly referred to as microwave and infrared,” explains Gianfelice Cinque, Principal Beamline Scientist for MIRIAM. “Since the turn of the millennium, interest has surged in spectroscopy studies at such frequencies because of new instrumentation capabilities.”
The broad spectrum of synchrotron radiation offered by Diamond can boost the longest wavelength emission in the millimetre region by operating the synchrotron in a dedicated mode, referred to as low alpha mode. This allows coherent synchrotron radiation to be emitted in the THz region as an intense photon flux which allows absorption measurements through relatively thick or highly absorbing materials. “We’re able to perform measurements in a 20 μm thick liquid cell filled with water and with low concentration (mM) of a protein,” continues Gianfelice, “which may not sound like much, but to do this over the wide infrared spectrum through one of the most absorbing IR materials like water is unique."