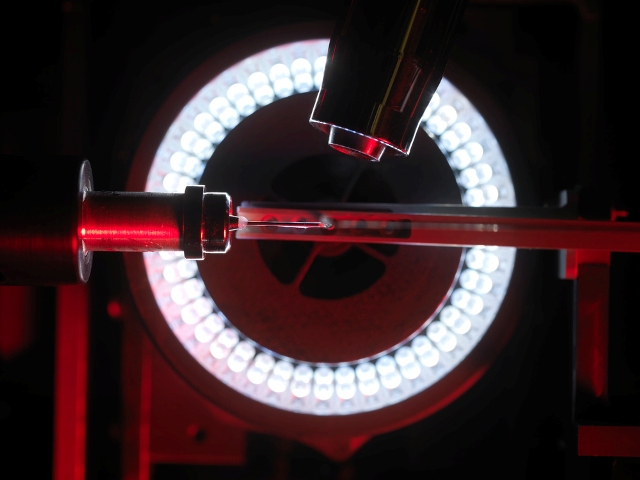
- View of the LptDE complex from outside of the bacterial cell with LptD shown in rainbow and LptE in grey The lipopolysaccharide (LPS) molecule that acts as a camouflage on the cell exterior enters the protein on the left hand side of the pore from the rear and extends out of the front. Finally LPS enters the outer membrane of the cell through an opening between the red and blue strands of LptD.
- @ Neil Paterson 2014
A group from the University of East Anglia, University of St Andrews and Diamond Light Source have made a breakthrough in the race to solve antibiotic resistance. Using Diamond, one of the UK’s most advanced scientific machines which produces a light 10 billion times brighter than the sun, they studied ‘superdrug’ bacteria in extreme detail to identify an innovative method of disabling bacteria and preventing antibiotic resistance.
The discovery doesn’t come a moment too soon. The World Health Organisation has warned that antibiotic-resistance in bacteria is spreading globally, with severe consequences. Even common infections, which have been treatable for decades, can once again kill. This breakthrough is a giant leap forward in the fight against superbugs.
Bacteria are able to infect their hosts because they camouflage themselves against the immune system. However, this new research, published today in the journal Nature, reveals how the bacteria construct this camouflage and opens the door to blocking the process through new classes of antibiotics.
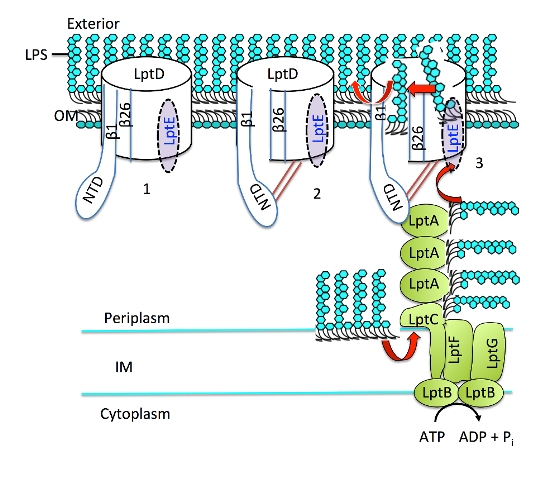
Researchers investigated Gram-negative bacteria, which cause a vast range of infections, including e-coli, salmonella, gonorrhea, pseudomonas, and meningitis. The outer surface of a Gram-negative bacterial cell acts as a disguising “cloak” that provides a barrier against toxic compounds such as antibiotics and camouflages the invading organism to evade detection and destruction by the body’s defences. Using the intense light produced by Diamond to study these bacteria at an atomic level, they were able to pinpoint the structure of the integral protein responsible for the final stage of creating the bacteria’s camouflage.
![]()
![]()