 | ||
Locking up radionuclides in minerals |
Radioactive technetium-99, Tc-99, is a contaminant in nuclear waste that is long-lived and can be highly mobile in the environment. It is therefore important to investigate how Tc environmental mobility can be limited as part of the decommissioning of the UK’s nuclear legacy. Mineral phases form in radioactive waste material due to a number of processes, including waste degradation and metal corrosion. An important phase formed in the absence of air is magnetite, Fe3O4, which is a corrosion product of steel. A combination of X-ray diffraction, electron microscopy, chemical extractions, and Extended X-ray Absorption Fine Structure Spectroscopy (EXAFS) using the Core EXAFS beamline (B18) at Diamond Light Source provided direct evidence that Tc(VII) was reduced and incorporated into the magnetite structure. Detailed structural information about the local bonding environment was obtained, confirming that the reduced Tc(IV) was incorporated into the structure by direct substitution of iron in the mineral phase over a large pH range. Crucially, the majority of the Tc-99 remains associated with the mineral when it is exposed to air and oxidised. This has important implications for the long-term irreversible retention of technetium in environments containing magnetite, potentially aiding in the storage of radioactive waste in deep underground geological disposal facilities.
Beamline B18 Scientific Highlight
Technetium-99 (Tc-99) is a long-lived radioactive fission product (half-life 2.1 x 105 years) present in many radioactive materials derived from nuclear fuel cycle operations and weapons production. Controlling and predicting the mobility and fate of radionuclides, including Tc-99, in contaminated land and radioactive waste disposal scenarios is key to delivering the UK’s national programmes in nuclear decommissioning and waste disposal. The long-term plan for management of higher activity radioactive waste is disposal within a deep geological repository utilising an engineered, multi-barrier system. For the intermediate level waste stream, the radioactive material is currently encapsulated within a cement grouted waste form and contained in a metal canister: at some point several tens of years in the future, it will be emplaced within the UK’s deep geological disposal facility. At this point it is envisaged the repository will be backfilled with cement when the facility is closed. Under oxidising conditions Tc(VII) is highly soluble (∼11 M) and environmentally mobile, existing as the pertechnetate (Tc) ion. Due to its high mobility under oxic conditions and long half-life, Tc-99 is a significant risk driving radionuclide for geological disposal of radioactive wastes. In addition, it also presents a significant challenge to remediation of the global legacy of radioactively contaminated land from fuel cycle operations and weapons production. However, in its reduced form, Tc(IV), it is sparingly soluble (10−8−10−9 M) and has very limited environmental mobility. Past work has shown that both abiotic1 (via Fe(II) bearing oxides e.g. magnetite, Fe3O4) and microbially mediated2 reduction processes where Fe(II) is produced are effective at reducing Tc(VII) thus limiting its mobility. However, research has also shown that when these systems are reoxidised the Tc is often partially remobilised. In this study, we focused on understanding the mechanism of Tc sequestration into magnetite in order to understand how to effectively limit Tc transport.
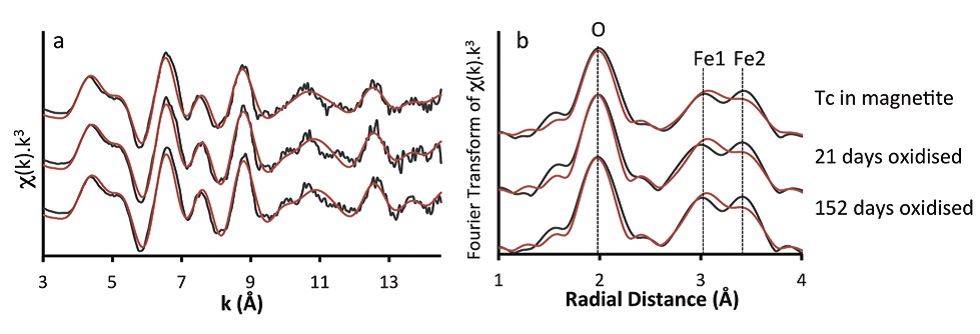
Figure 1: Technetium K-edge XAS spectra from Tc-99 coprecipitated with magnetite and subsequent air oxidation in Intermediate Cement Leachate (pH 12.5). (a) k3-weighted EXAFS; (b) Fourier
transform of k3-weighted EXAFS. The spectra are almost identical indicating Tc(IV) is retained in the same incorporation site within the crystal structure throughout the formation and oxidation
process.
Recent computer modeling studies3 have suggested Tc(IV) can directly substitute for octahedral coordinated Fe within the structures of iron oxides i.e. hematite (α-Fe2O3). If Tc(IV) can be incorporated into a mineral structure, it may be possible to essentially lock up the Tc, and prevent oxidative release. Iron oxides are important within geodisposal scenarios as they are key products of steel corrosion and are also prevalent in soils and sediments. In particular, magnetite is a dominant and persistent corrosion product formed under the anaerobic conditions that are expected to predominate in a deep radioactive waste disposal facility4. Experiments were performed to determine whether Tc-99 as pertechnetate could be reductively incorporated into the structure of magnetite during crystallisation. The mechanism of incorporation within the magnetite structure, and the effect of oxidative perturbation on the Tc-99 speciation and solubility were also investigated. The synthesis and oxidation of magnetite in the presence of Tc-99 were performed under conditions relevant to the anaerobic and alkaline (pH 10.5 – 13.5) conditions within a geodisposal facility which uses cement. In order to determine the speciation of Tc-99 associated with the mineral samples Tc K-edge X-ray Absorption Spectra (XAS) were collected using the facilities on beamline B18. The Tc Extended X-ray Absorption Fine Structure (EXAFS) data collected were very high quality providing unparalleled information on the local bonding environmental of Tc associated with magnetite and the oxidation products.
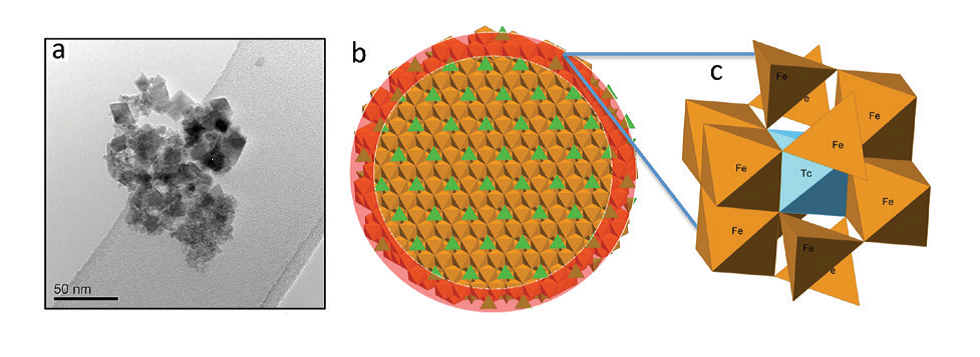
Figure 2: (a) TEM image of magnetite crystallised in Intermediate Cement Leachate (pH 12.5); (b) model of magnetite nanoparticle illustrating the enrichment of Tc near the surface of the particle (highlighted in red); (c) model showing the mechanism of Tc incorporation into magnetite.
Following crystallisation of magnetite, chemical analyses indicated all the Tc was associated with the solid phase. X-ray Absorption Near Edge Structure (XANES) and EXAFS data showed that Tc(IV) was directly incorporated into the magnetite structure (Fig. 1). Detailed analysis of the EXAFS data showed that the Tc directly substitutes for octahedrally coordinated Fe within the iron oxide. However, the Tc-Fe coordination numbers were lower than those expected for magnetite (4.4 – 4.7 versus 6). In nanoparticulate systems, surface effects may result in lower coordination numbers; atoms residing in the near-surface of a particle will have a lower coordination in the metal−metal shells than those in the particle core, and this suggests that the Tc was concentrated near the surface of the magnetite nanoparticles (5-20 nm in size, Fig. 2). This was supported by dissolution experiments which showed an initial rapid release of near-surface bond Tc as the particles dissolved under acidic conditions. The ionic radii of Tc(IV) and Fe(III) are identical at 0.785 Å, and so, the magnetite structure should accommodate Tc(IV) with little distortion. There is an excess charge associated with Tc(IV) substituting for Fe(III), and this may cause the slightly lengthened Tc−Fe1 atomic distance (∼3.05 Å) observed from the EXAFS fitting in our samples compared to the Fe−Fe distance in magnetite (2.97 Å). However, the Tc-Fe2 distance was identical to the Fe-Fe distance in magnetite (i.e. 3.48 Å). On reoxidation of the magnetite in air, an extreme treatment when considering deep geological disposal where the subsurface will likely be anoxic in the long term, the Fe(II) bearing phase was significantly reoxidised and gradually transformed into a mixture of maghemite (g-Fe2O3) and goethite (FeOOH). During this process less than 40% of the Tc was rereleased into solution and it was predominantly from the outer part of the oxide particles, indicating that most of the incorporated Tc was recalcitrant to oxidative dissolution despite the magnetite oxidising to maghemite and goethite. XANES and EXAFS (Fig. 1) analysis showed that all the solid associated Tc remained as Tc(IV) and continued to be substituted for octahedral coordinated Fe within the reoxidised magnetite/maghemite structure.
Source publication:
Marshall, T. A., Morris, K., Law, G. T. W., Mosselmans, J. F. W., Bots, P., Parry, S. A. & Shaw, S. Incorporation and Retention of 99-Tc(IV) in Magnetite under High pH Conditions. Environmental Science & Technology 48, 11853-11862, doi:10.1021/es503438e (2014).
References:
1. Scott, T. B., Allen, G. C., Heard, P. J. & Randell, M. G. Reduction of U(VI) to U(IV) on the surface of magnetite. Geochimica Et Cosmochimica Acta 69, 5639-5646, doi:10.1016/j.gca.2005.07.003 (2005).
2. Burke, I. T. et al. Effects of progressive anoxia on the solubility of technetium in sediments. Environmental Science & Technology 39, 4109- 4116, doi:10.1021/es048124p (2005).
3. Skomurski, F. N., Rosso, K. M., Krupka, K. M. & McGrail, B. P. Technetium Incorporation into Hematite (alpha-Fe2O3). Environmental Science & Technology 44, 5855-5861, doi:10.1021/es100069x (2010).
4. Williamson, A. J. et al. Microbial reduction of U(VI) under alkaline conditions: implications for radioactive waste geodisposal. Environmental Science & Technology 48, 13549-13556, doi:10.1021/es5017125 (2014).
Funding acknowledgement:
This work has been funded as part of the UK Natural Environment Research Council (NERC) BIGRAD consortium through consortium grant NE/H007768/1.
Corresponding author:
Dr Sam Shaw, University of Manchester, [email protected]


 A brighter light for science
A brighter light for science
