____________________________________
Industrial Liaison Group:
Tel: +44 (0) 1235 778797
E-mail: [email protected]
The ability to modulate drug delivery at therapeutically effective doses over a sustained period of time, in vivo, is very challenging. In the case of poorly water-soluble drugs this requires a carefully designed matrix to manage and maintain their controlled release.
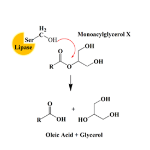
Lipid cubic phase carriers offer an effective way to transport both small molecules and larger proteins through oral and parenteral routes (those outside of the digestive tract), as well as local delivery via subcutaneous and intramuscular routes. Complex interactions between the drug and the lipid matrix govern the release profile; for hydrophilic drugs, release can be very fast. The carriers can also be compromised by naturally occurring lipolytic enzymes which act to break down the lipid microstructure.
A team from the University of Limerick, the University of Bristol and COOK
Ireland Ltd have developed an approach in which an enzyme inhibitor is
incorporated into the lipid network forming the carrier system to regulate
carrier degradation and thereby control the release profile.
Polarising optical microscopy has been used to detect the presence of liquid crystalline structures; however, a more advanced analytical technique was necessary to distinguish between different mesophases.
The scientific team used small angle X-ray scattering (SAXS) on beamline B21 to analyse the lipid microstructures. A series of experiments were designed to investigate the microstructures formed and how these varied with inhibitor and API loading and to track changes over a period of ageing from 1 hour to 30 days. The high intensity of the synchrotron X-rays allowed rapid measurements, essential for ageing experiments, and enabled the team to establish which mesophases were present.
SAXS investigations showed exactly which lipid mesophase(s) were present in each sample. The measurements showed that the addition of inhibitor and API did not negatively affect the microstructure of the matrix. The release kinetics are governed by the particular mesophase formed so a detailed understanding of the microstructure allowed the team to create a tuneable lipid release system.
“Cook Medical New Ventures focuses on transforming patient care
through technology generation. In our collaboration with the University of
Limerick to develop lipid based carrier systems we needed a sensitive
measurement technique to characterize the lipid microstructures
over time. Gaining access to the world-leading facilities and staff at
Diamond Light Source was key in completing this work and advancing
our understanding of this technology for future patient treatments.”
David Murray, COOK Medical


“The high resolution data collected at the B21 beamline helped us to properly characterise our lipid drug delivery formulations which will enable their development as stable delivery vehicles for drug candidates that exhibit challenging solution behaviours. The custom viscous sample holders designed by the Diamond scientists made analysis of our sticky, tacky, lipid formulations straight forward and simple.
We are very grateful for the attentive support and assistance from the Diamond staff and scientists.”
Michele Dully, Dept. Chemical Sciences, Bernal Institute at theUniversity of Limerick


Diamond Light Source is the UK's national synchrotron science facility, located at the Harwell Science and Innovation Campus in Oxfordshire.
Copyright © 2022 Diamond Light Source
Diamond Light Source Ltd
Diamond House
Harwell Science & Innovation Campus
Didcot
Oxfordshire
OX11 0DE
Diamond Light Source® and the Diamond logo are registered trademarks of Diamond Light Source Ltd
Registered in England and Wales at Diamond House, Harwell Science and Innovation Campus, Didcot, Oxfordshire, OX11 0DE, United Kingdom. Company number: 4375679. VAT number: 287 461 957. Economic Operators Registration and Identification (EORI) number: GB287461957003.