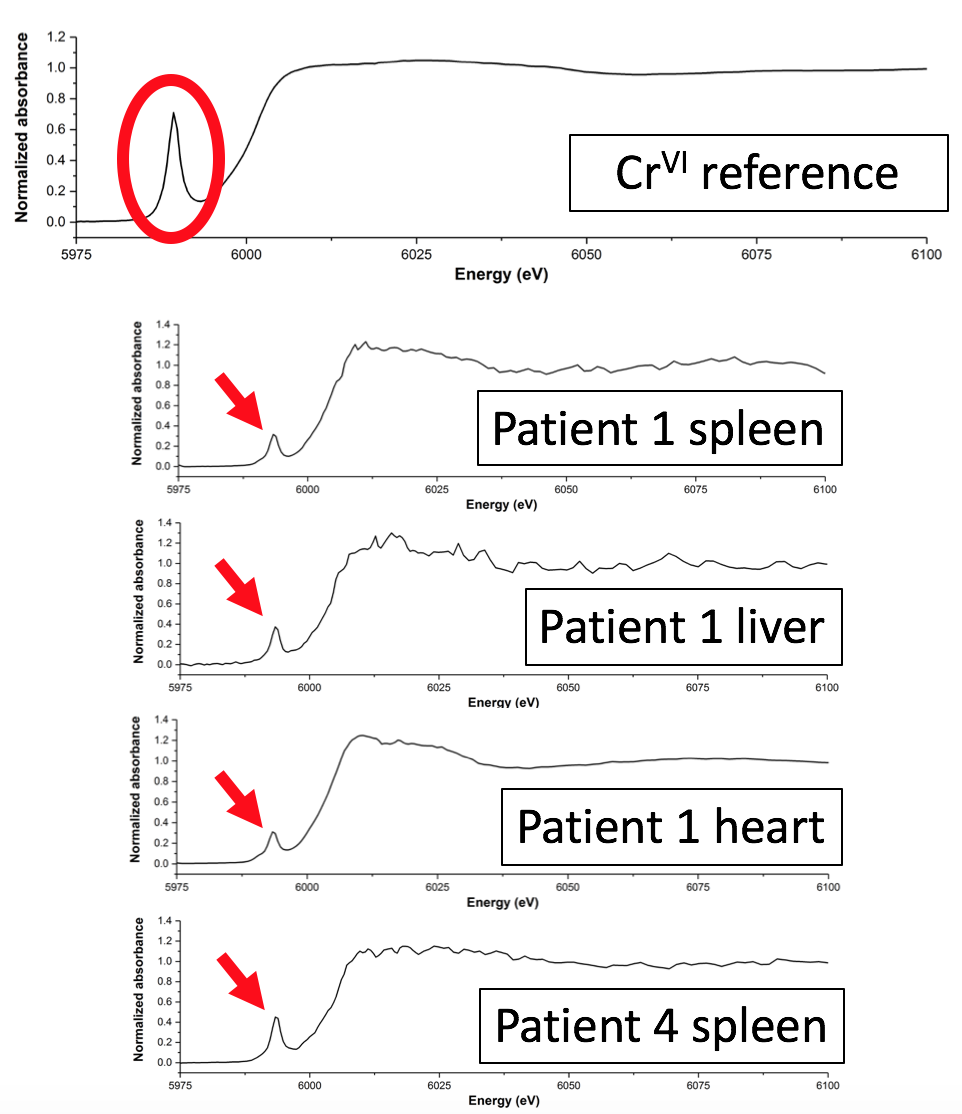
The hip replacement is considered to be one of the most successful orthopaedic interventions, with 75,000 performed each year by the NHS alone. However, the implants used to replace hips contain metals, such as chromium and cobalt, which are potentially toxic and which can be deposited into tissues around the implant site due to wear and corrosion. A team of researchers used X-ray absorption spectroscopy (XAS) on the I18 beamline to show that these metals can also find their way into organ tissues. Their results suggest that chronic diseases, such as diabetes, may create conditions in which mildly toxic trivalent chromium (CrIII) particles from replacement joints are reoxidised within the body to form carcinogenic hexavalent chromium (CrVI). Their results have been published in the Journal of Trace Elements in Medicine and Biology.
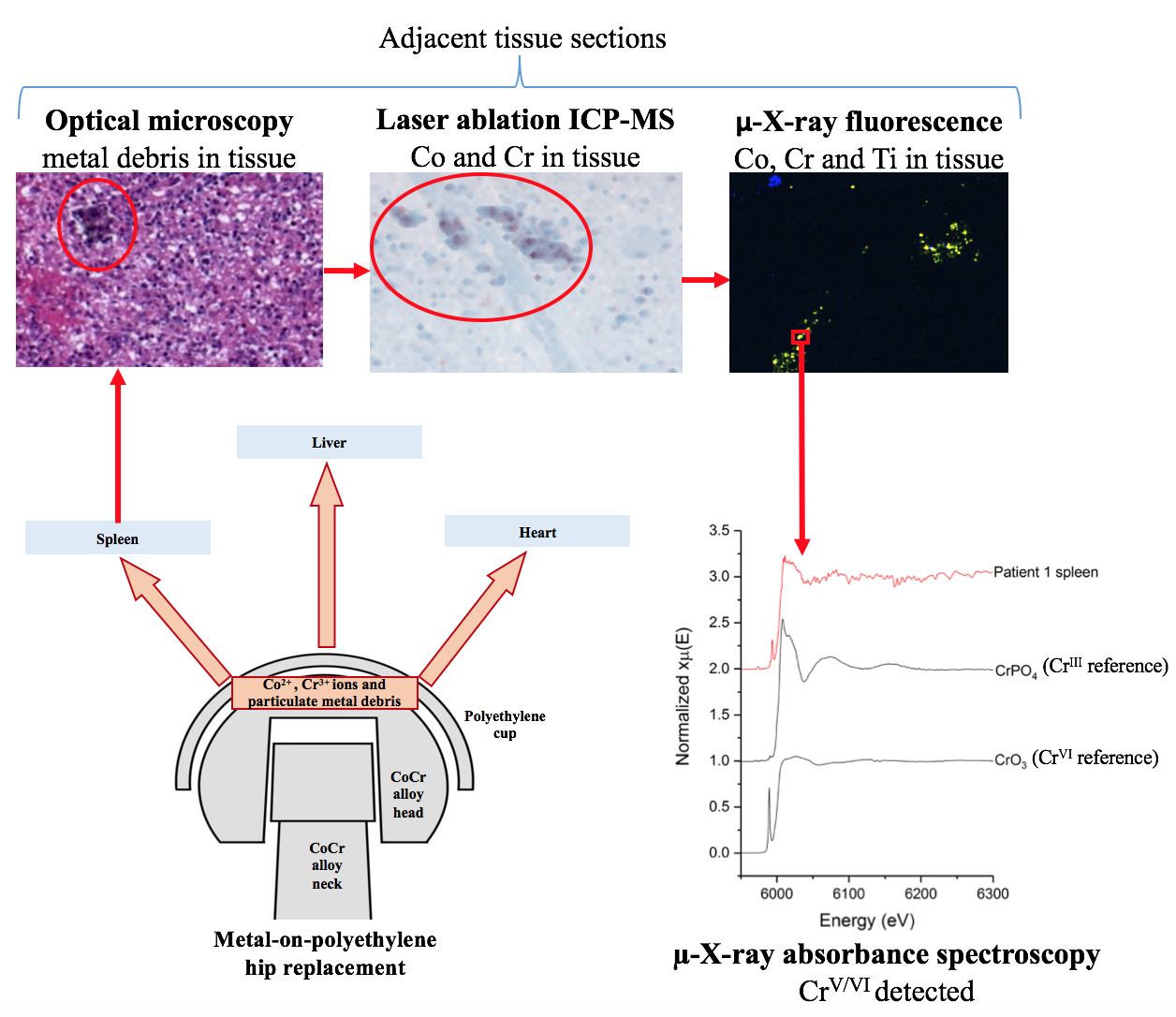
Figure 1: Overview of the study.
Metals on the move
Hip replacement operations have over a hundred years of history, with the earliest recorded attempts taking place in Germany in 1891. Pioneering English surgeon George McKee was the first to use metal-on-metal (MOM) hip joints on a regular basis, in the 1950s, and Sir John Charnley at the Manchester Royal Infirmary pioneered the modern hip replacement technique in the early 1960s. MOM implants became unpopular in the 1970s when follow-up operations found metal particles in the tissues around the implant. Metal-on-polyethylene (MOP) implants are now the most widely used type.
Swiatkowska I et al. Synchrotron analysis of human organ tissue exposed to implant material. Journal of Trace Elements in Medicine and Biology 46, 128-137 (2018). DOI:10.1016/j.jtemb.2017.12.007.
Diamond Light Source is the UK's national synchrotron science facility, located at the Harwell Science and Innovation Campus in Oxfordshire.
Copyright © 2022 Diamond Light Source
Diamond Light Source Ltd
Diamond House
Harwell Science & Innovation Campus
Didcot
Oxfordshire
OX11 0DE
Diamond Light Source® and the Diamond logo are registered trademarks of Diamond Light Source Ltd
Registered in England and Wales at Diamond House, Harwell Science and Innovation Campus, Didcot, Oxfordshire, OX11 0DE, United Kingdom. Company number: 4375679. VAT number: 287 461 957. Economic Operators Registration and Identification (EORI) number: GB287461957003.