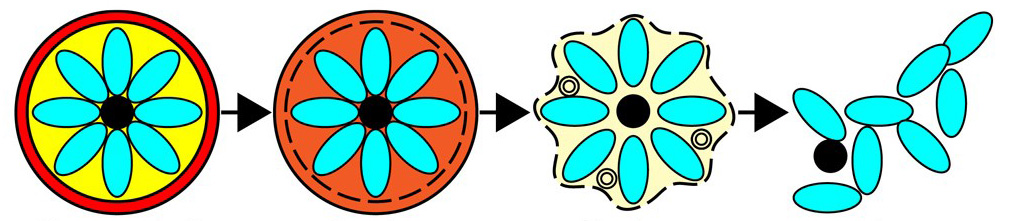
Figure 1: A pictorial representation of the four primary stages of egress.
Parasitic escape
Upon host infection with a malaria parasite, the infective material initially travels via the bloodstream to the liver where it proliferates to produce thousands of daughter cells. These new cells, known as merozoites, return to the bloodstream where they infect red blood cells to initiate the blood life cycle of the parasite.
During this phase, the parasites multiply rapidly inside red blood cells within parasitophorous vacuoles, and the daughter cells exit the red blood cells via egress. To escape their containment and further the infection, the merozoites must break through two membranes: the parasitophorous vacuole membrane (PVM) and the red blood cell membrane. The exact mechanism of this double rupture, in particular the order and timings of these breakages, were unclear.
A team of scientists based in London, hailing from Birkbeck College, Kings College, London School of Hygiene & Tropical Medicine and the Francis Crick Institute, set about clarifying the stages involved in this latter part of the malaria life cycle.
Intact cells
Parasites inside red blood cells were synchronised so that they were at a similar stage of the infection cycle. Two pharmacological blockers were used that could stall egress at different stages, which enabled the team to trap intermediates in the process. At critical points, the team prepared samples for X-ray tomography by plunge freezing in liquid ethane. At Diamond’s B24, tilt series were collected on the UltraXRM-S/L220c X-ray microscope (Zeiss) with a 1024 PixisB CCD camera (Princeton Instruments) and a 40 nm zone plate with X-rays of 500 eV. These tilt series were then reconstructed to provide a 3D reconstruction of the red blood cells.
Professor Helen Saibil, Professor of Structural Biology at Birkbeck and lead investigator of the study explained why B24 was chosen: “With electron tomography you can only look at thin sections, so you miss most of the cell volume. Cryo soft X-ray tomography allowed us to look at frozen, hydrated intact cells, so that we could see the whole cells, along with their major membranes”.
As well as soft-X-ray tomography, the team used an array of other techniques to observe egress, such as video microscopy, electron tomography and electron energy loss spectroscopy.
Different timings
Using B24, the team observed the breakage of both the PVM and the red blood cell membrane to piece together the intermediate stages of egress. Prof Saibil explained their findings: “Overall, we saw that the timings of the breakages were quite different to those reported in the literature. We found that the vacuole membrane became leaky much earlier than had been previously reported, so an earlier start to the process was identified. We also saw that the red cell collapsed only at the very end, just during the final seconds of egress, whereas it was previously thought that this step happened gradually”.
As well as clarifying the timings and nature of the membrane breakdowns, the results hint towards an unknown triggering event happening upstream of egress. This work will continue with the assistance of the genome editing CRISPR/Cas9 technology to genetically alter the main players of egress and fully characterise this process. Understanding the exact events during this critical phase of infection could enable new medicines to be developed to counter malaria infections in the future.
Figure 2: The two intermediate stages of egress, where the vacuole (yellow) is densely packed with merozoites (cyan) all housed within the erythrocyte membrane (red).