The crystal structure of a crucial regulator of glucose metabolism has been revealed for the first time at Diamond Light Source. The momentous discovery, recently published in Nature, could allow a new generation of diabetes drugs to be designed.
Type 2 diabetes is a global health concern, and is characterised by an inability to sense and respond to rising levels of glucose. Glucagon-like peptide 1 (GLP-1) is an important hormone that is critical to the metabolism of glucose. This hormone is released from the gastrointestinal tract in response to food and it promotes the secretion of insulin from the pancreas. Stimulation of the GLP-1 receptor is a means of treating type 2 diabetes, but although there are several GLP-1 agonists approved for the treatment of type 2 diabetes, they are expensive and administered by injection only. Alternatives, such as small molecules or smaller peptides that can be taken orally are much needed.
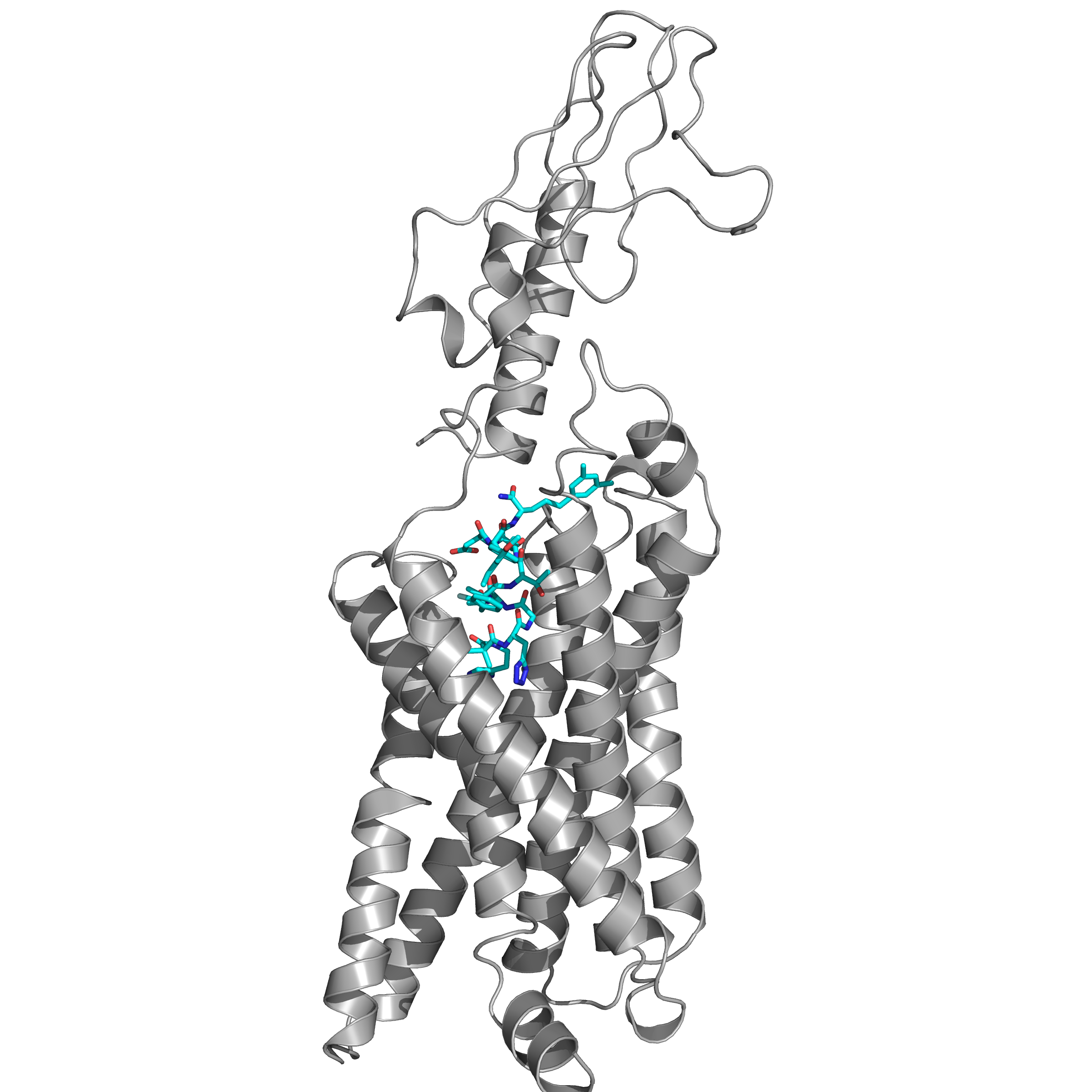
Figure 1: Structure of human GLP-1 receptor in complex with an agonist peptide
Glucose control
Type 2 diabetes is a condition associated with poor glucose control. People with type 2 diabetes do not respond adequately to a hormone known as GLP-1 which stimulates the release of insulin from the pancreas in response to food. GLP-1 stimulation is a well established treatment strategy to manage type 2 diabetes, yet the licensed drugs are long peptides and need to be injected. An oral drug in this setting would be revolutionary.
Diamond Light Source is the UK's national synchrotron science facility, located at the Harwell Science and Innovation Campus in Oxfordshire.
Copyright © 2022 Diamond Light Source
Diamond Light Source Ltd
Diamond House
Harwell Science & Innovation Campus
Didcot
Oxfordshire
OX11 0DE
Diamond Light Source® and the Diamond logo are registered trademarks of Diamond Light Source Ltd
Registered in England and Wales at Diamond House, Harwell Science and Innovation Campus, Didcot, Oxfordshire, OX11 0DE, United Kingdom. Company number: 4375679. VAT number: 287 461 957. Economic Operators Registration and Identification (EORI) number: GB287461957003.