Fischer-Tropsch synthesis is a well-known process used to convert hydrogen and carbon monoxide to hydrocarbons. The process can be used to produce short, long hydrocarbon chains or heavy waxes, and so catalysts are important to promote certain products or increase the activity of the reaction process.
Although chemists know that certain Fischer-Tropsch catalysts behave in particular ways, to date the specific structural features that cause this have been largely unknown. However in a paper recently published in Science Advances researchers at Diamond Light Source, University College London, and other collabortaors from the UK Catalysis Hub, have shone light on this. They used a combination of different X-ray techniques concurrently to infer how cobalt catalysts changed at both the micro- and nanoscale throughout the Fischer-Tropsch process. Their novel technique allowed them to obtain spatial insight of the catalysts’ evolution throughout the reaction process in conditions approaching those used in industry.
The team’s results showed that even for catalysts with identical atomic make up, different nano- and microstructures are exhibited due to very small modifications in how the catalyst was synthesised. This in turn causes differences in the selectivity and activity of the catalyst. They hope this work can serve as a case study for how to determine the structural make up of catalysts under real operating conditions, and that their technique can be extended to investigate other catalytic systems.
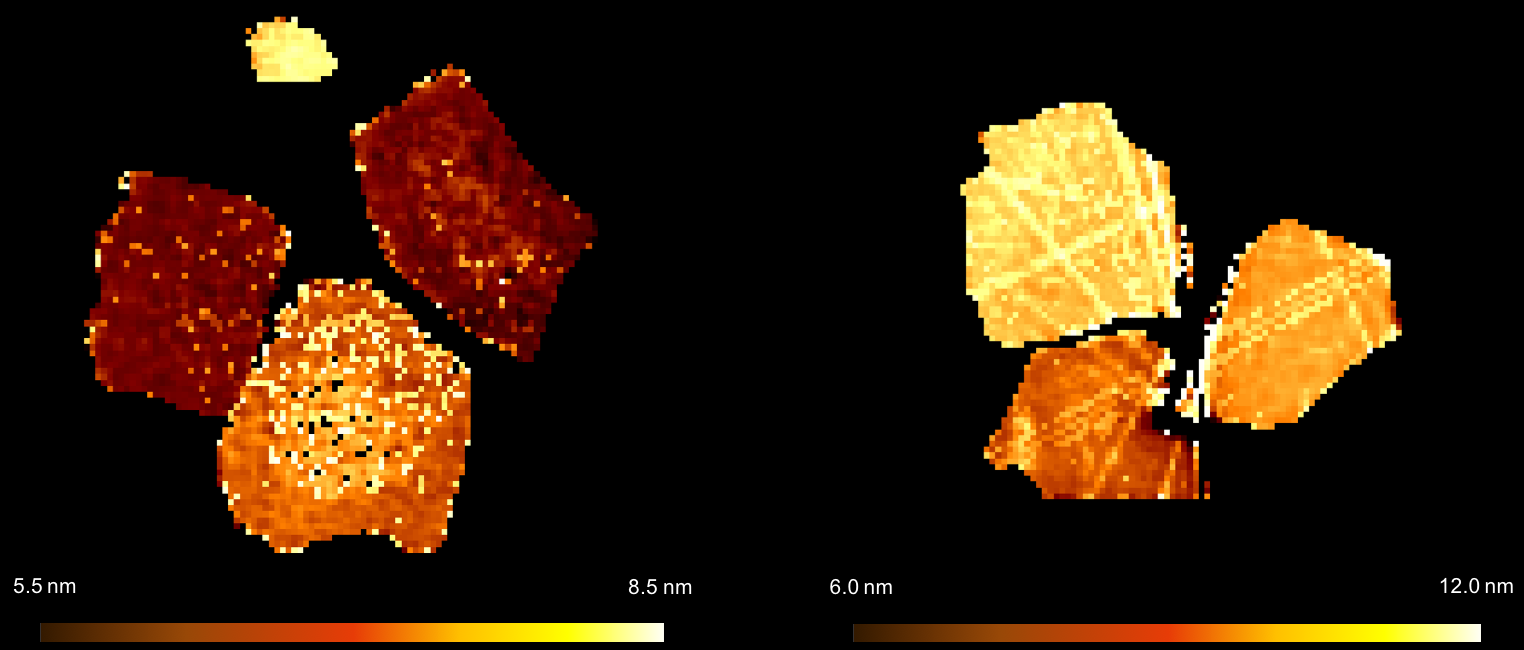
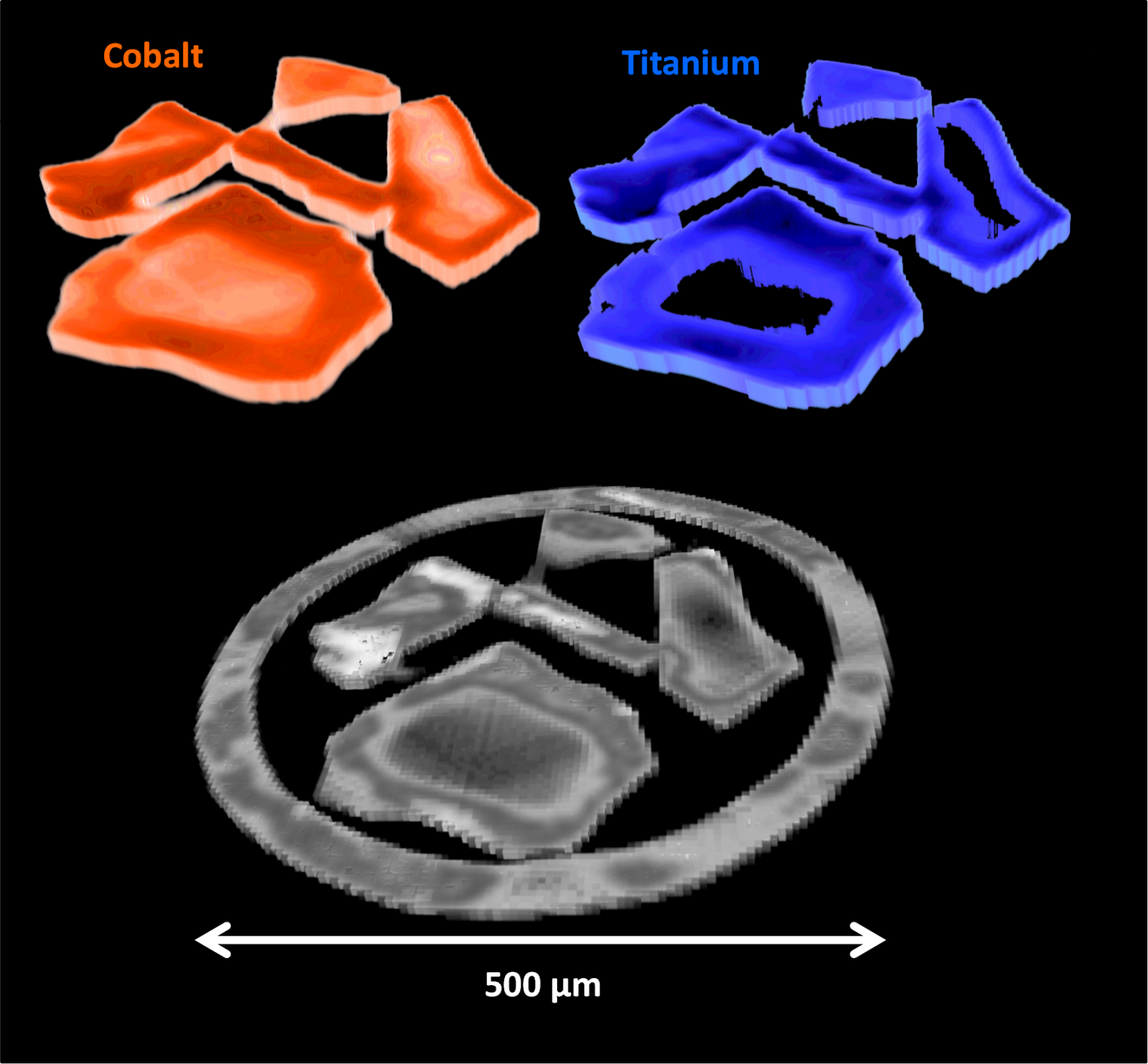
To find out more about the I18 beamline, or to discuss potential applications, please contact Principal Beamline Scientist Prof Fred Mosselmans: fred.mosselmans@diamond.ac.uk
Price SWT et al. Chemical imaging of Fischer-Tropsch catalysts under operating conditions. Sciences Advances, 3, 3 (2017).
Diamond Light Source is the UK's national synchrotron science facility, located at the Harwell Science and Innovation Campus in Oxfordshire.
Copyright © 2022 Diamond Light Source
Diamond Light Source Ltd
Diamond House
Harwell Science & Innovation Campus
Didcot
Oxfordshire
OX11 0DE
Diamond Light Source® and the Diamond logo are registered trademarks of Diamond Light Source Ltd
Registered in England and Wales at Diamond House, Harwell Science and Innovation Campus, Didcot, Oxfordshire, OX11 0DE, United Kingdom. Company number: 4375679. VAT number: 287 461 957. Economic Operators Registration and Identification (EORI) number: GB287461957003.