In conventional electronic computers information is stored as a series of bits which are held within memory as either a high voltage or a low voltage, designated as the digits of 1 or 0 respectively. This simple coding system has served as the foundation of computing over the past three quarters of a century. With the need for denser data storage, and the advent of quantum computing, there has been a drive to use the quantum state of individual molecules (or the discrete spin state) to store information, rather than the voltage< states of gates within microchips, where the digital 1 or 0 can be designated as the molecule having a spin up state (S = ½) or spin down state (S = -½). Molecular {Cr7Ni} rings have been studied widely as possible two-level systems that can act as qubits (quantum bits) as they have the advantage over other potential candidates in that they can be readily combined into large supramolecular assemblies (large wellordered structures containing several molecules). In this study single-crystal X-ray diffraction has been used, on the Small-Molecule Single-Crystal Diffraction beamline (I19) at Diamond Light Source, to characterise the structures of a range of these assemblies.
The idea that the electron spin in anti-ferromagnetically coupled molecules could be used to prepare qubits for quantum information processing was first proposed by the Loss group2. One family of molecules that can be used to act as qubits are based on {Cr7Ni} rings, which contain an octagon of metal sites bridged by carboxylates and fluorides, producing a structure where there is anti-ferromagnetic exchange between the S = 3/2 CrIII ions and the S = 1 NiII ions3. This results in an S = ½ ground state, and it has been shown these ground states have coherence times that are sufficiently long that it is not entirely unreasonable to propose them as qubits4.
The rings are templated about a cation3, which is typically a protonated secondary amine. This has allowed the preparation of hybrid inorganic-organic [2]- and [3]-rotaxanes, where the distance between the S = ½ rings through organic chemistry can be controlled, and this in turn controls the interaction between these units – which is an important consideration if the molecules are to be used as qubits5. However even a [3]-rotaxane would contain only two qubits, and to implement any useful quantum algorithm many more qubits would be needed per assembly. The present work concerns designing routes that could lead to larger assemblies1.
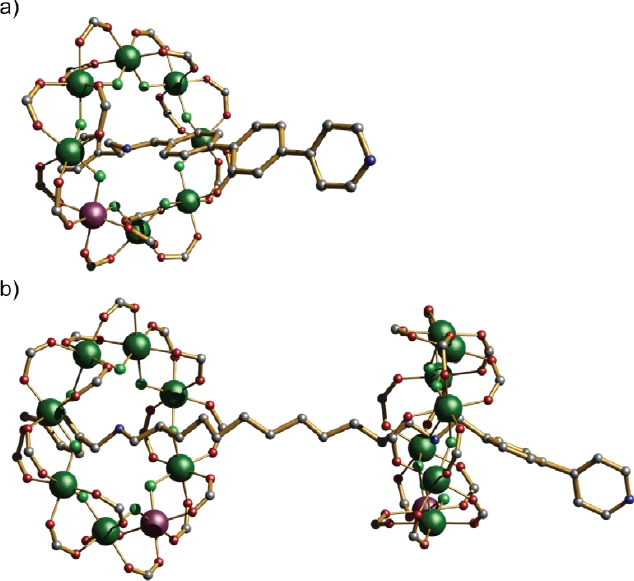
The [2]- and [3]-rotaxanes can be terminated with pyridyl groups on the organic thread (Fig.1) and this produces rotaxanes that can then be used in a subsequent chemical step as ligands for simple coordination compounds. The size of the eventual assembly is then controllable either by the choice of both the rotaxane ligand used, and the number of vacant coordination sites on the coordination complexes. The distance between units can also be varied by organic chemistry, e.g. by varying the number of phenyl-groups present in the thread.
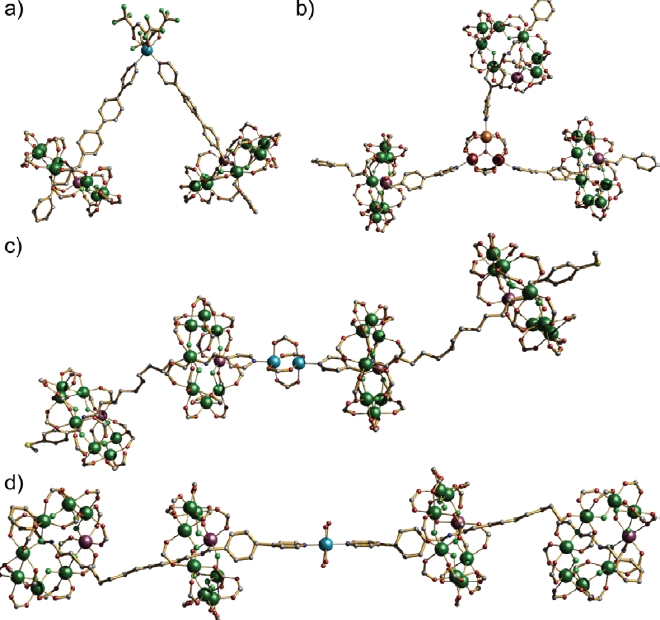
This is easily illustrated by reacting either the [2] or [3]-rotaxane ligands with monometallic complexes with two vacant coordination sites, e.g. Cu(NO3)2.xH2O or Cu(hfac)2, or dimetallic complexes with two vacant sites, e.g. [Cu2(O2CtBu)4] or trimetallic complexes with three vacant sites, e.g. [FeIII2CoIIO(O2CtBu)6(H2O)3]. The results are, in some ways, entirely predictable. If a [2]rotaxane is used as ligand, and the coordinated complex has two sites a [3] rotaxane results (Fig. 2a). If a [2]rotaxane is used with a three-connector at the centre, a [4]rotaxane results (Fig. 2b). When a [3]rotaxane such as that shown in Fig. 1b is used, then linking two leads to a [5]rotaxane (Fig. 2c and 2d).
While the formation of these oligo-rotaxanes is predictable, proving the result is extremely challenging. The compounds are very large and crystallisation is difficult. The molecules crystallise with as many as 64 disordered tertiary butyl groups per molecule, which leads to crystals that are weakly diffracting. The use of synchrotron radiation is mandatory to obtain sufficient diffraction to solve these structures. One molecule does not crystallise; this is the [7]rotaxane which results from linking three [3]rotaxanes via an oxo-centred metal triangle. This structure can be predicted, and is supported by measurements using small angle X-ray sources, again using synchrotron radiation for some of these measurements.
References:
Funding acknowledgement:
This work was supported by the EPSRC(UK), the European Commission (Marie Curie Intra-European Fellowship to A.F. (300402) and J.F.S. (622659). We thank Diamond Light Source for access to synchrotron X-ray facilities, and specifically Drs Nuntaporn Kamonsutthipaijit, Dmitry Kondratiuk and Sophie Rousseaux for assistance with SAXS experiments and Drs Dave Allan and Harriet Nowell for help with single crystal measurements.
Corresponding authors:
Dr Antonio Fernandez, Dr Jesus Ferrando-Soria, Dr Iñigo J. Vitorica-Yrezabal, Dr Grigore A. Timco and Prof Richard E. P. Winpenny, The University of Manchester, [email protected]
Related publication:
Fernandez A, Ferrando-Soria J, Pineda, EM, Tuna F, Vitorica-Yrezabal IJ, Knappke C, Ujma J, Muryn C, Timco G, Barran P, Ardavan, A R, Winpenny. Making Hybrid [n]-Rotaxanes as Supramolecular Arrays of Molecular Electron Spin Qubits. Nature Communications 7, 10240, doi:10.1038/ncomms10240 (2016).
Publication keywords:
Inorganic chemistry; Quantum information; Qubits; Supramolecular chemistry
Diamond Light Source is the UK's national synchrotron science facility, located at the Harwell Science and Innovation Campus in Oxfordshire.
Copyright © 2022 Diamond Light Source
Diamond Light Source Ltd
Diamond House
Harwell Science & Innovation Campus
Didcot
Oxfordshire
OX11 0DE
Diamond Light Source® and the Diamond logo are registered trademarks of Diamond Light Source Ltd
Registered in England and Wales at Diamond House, Harwell Science and Innovation Campus, Didcot, Oxfordshire, OX11 0DE, United Kingdom. Company number: 4375679. VAT number: 287 461 957. Economic Operators Registration and Identification (EORI) number: GB287461957003.