'Chirality’ is derived from the Greek word for hand, and is given to describe something that cannot be superimposed on its mirror image form. Left and right hands can be described as chiral because they are non-superimposable mirror images – if you hold out both hands with palms facing upwards and lay them directly on top of each other, the thumb of one hand would be over top of the little finger.
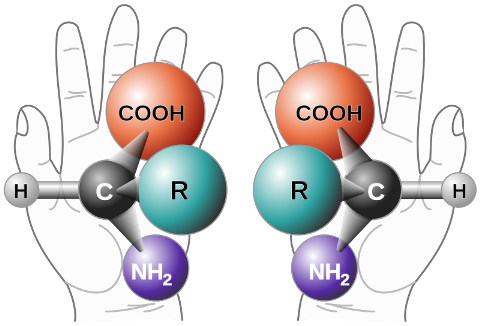 Demonstrating the concept of chirality with two mirror-related chiral amino acid molecules.
Demonstrating the concept of chirality with two mirror-related chiral amino acid molecules.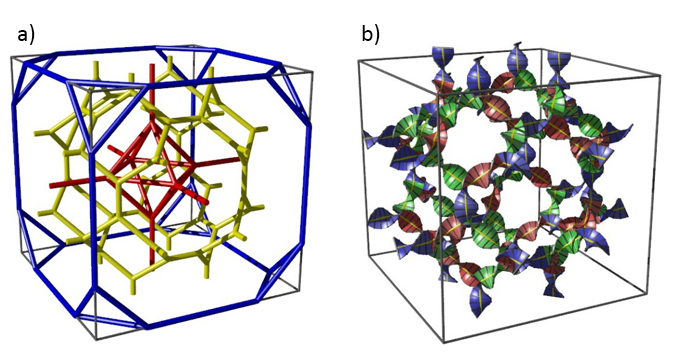
Figure: (a) Unit cell of the Im3m cubic liquid crystalline phase; the three networks are coloured blue, yellow and red.1 (b) The middle network (yellow in (a)) represented as twisted ribbons with the black rods symbolising the rod-like molecules.
Dressel et al. Dynamic Mirror-Symmetry Breaking in Bicontinuous Cubic Phases. Angew. Chem. Int. Ed. 53, 13115-20 (2014). DOI: 10.1002/anie.201406907
Diamond Light Source is the UK's national synchrotron science facility, located at the Harwell Science and Innovation Campus in Oxfordshire.
Copyright © 2022 Diamond Light Source
Diamond Light Source Ltd
Diamond House
Harwell Science & Innovation Campus
Didcot
Oxfordshire
OX11 0DE
Diamond Light Source® and the Diamond logo are registered trademarks of Diamond Light Source Ltd
Registered in England and Wales at Diamond House, Harwell Science and Innovation Campus, Didcot, Oxfordshire, OX11 0DE, United Kingdom. Company number: 4375679. VAT number: 287 461 957. Economic Operators Registration and Identification (EORI) number: GB287461957003.