Related publication:
Grundner S, Markovits MAC, Li G, Tromp M, Pidko EA, Hensen EJM, Jentys A, Sanchez-Sanchez M, Lercher JA. Singlesite trinuclear copper oxygen clusters in mordenite for selective conversion of methane to methanol. Nature Communications 6, 7546, doi:10.1038/ncomms8546 (2015).
Keywords:
Methane; Methanol; Oxidation; Catalysis; Characterisation; XAS; Calculation.
Spectroscopy Village | Beamline B18
Copper exchanged zeolites have been extensively studied for a broad range of industrially important reactions, including the selective partial oxidation of methane (CH4) to methanol (CH3OH). Detailed spectroscopy studies have been applied in literature to understand the Cu speciation and catalytically active Cu site in the zeolite structure. Multinuclear and especially dimeric copper clusters are typically the proposed active species. However, in the majority of these studies there is clear evidence for the presence of spectator species, i.e. multiple inactive Cu sites, with only a minority of catalytically active ones.
Here, a single-site copper-oxygen cluster was prepared and characterised in zeolite mordenite for the selective conversion of CH4 to CH3OH. The choice of mordenite with a high concentration of Al in the side pockets, together with an optimised synthetic approach for Cu exchange, allowed preparation of Cu-MOR materials1 and led to a significantly higher activity in CH4 activation than those reported in the literature for analogous systems. A linear dependence of the activity on the Cu concentration was observed for a large series of samples with different Cu loading. The stoichiometry of one converted CH4 molecule per three Cu atoms for these Cu-MOR materials with various Si/Al ratios demonstrated that it is possible to develop a Cu zeolite with only one type of active site and without any inactive Cu spectators.

Figure 1: Comparisonof the k2-weighted Fourier transformed EXAFS at the Cu Kedge of the Cu-MOR zeolite activated in O2 at 723 K and EXAFS simulation of an intra-zeolite a) binuclear [Cu(μ-O) Cu]2+ and c) trinuclear [Cu3(μ-O)3]2+ complex with b, d) the corresponding k2- weighted experimental EXAFS oscillations and their simulation using the DFT computed model. Color key: measured spectra (red lines), simulated spectra (black lines).
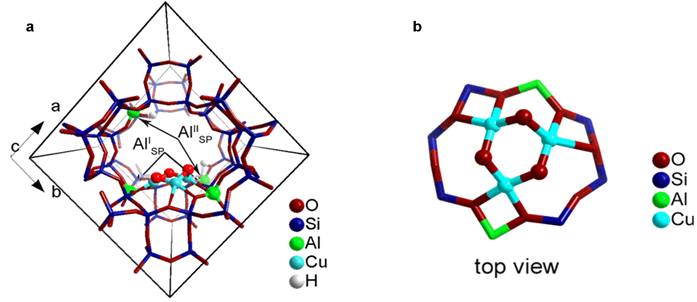
Figure 2: a) Location and b) structure of [Cu3(μ-O)3]2+ cluster in MOR zeolite model predicted by periodic DFT calculations (Si/Al=11). The zeolite model contained paired (type I) and isolated (type II) Al atoms located at the pore mouth of the side pocket. The cluster is stabilised by two anionic centres due to AlI lattice sites at the entrance of the MOR side-pocket so that the extra-framework oxygens responsible for the initial C-H activation are pointing towards the main channel of MOR. The charge due to the remaining AlII is compensated by acidic protons resulting in Bronsted acid site formation.
In order to investigate the nature of the Cu species, in situ X-ray Absorption Fine Structure (XAFS) spectroscopy in combination with infrared (IR) and ultraviolet-visible (UV-Vis) spectroscopies and density functional theory (DFT) calculations was performed on the activated Cu-MOR sample (activated in O2 at 723K). The reactivity data point to a uniform nature of the Cu site and hence the XAFS data should yield unambiguous information on the structure of the active clusters.
Figures 1a and 1b compare the k2-weighted Fourier Transform of the Cu K-edge Extended X-ray Absorption Fine Structure (EXAFS) data with a dimeric [Cu(μ-O)Cu]2+ cluster as previously proposed in literature (and optimised in the MOR structure using DFT). The experimental data significantly deviate from the dimeric model, especially at larger interactomic distances, pointing to presence of additional Cu···Cu contributions. Moreover, a dimeric Cu cluster is expected to display strong multiple scattering paths (from Cu-O-Cu, as in CuO), which is not visible in the experimental data. In Fig. 1c,d, the experimental EXAFS data was refined using a trimeric Cu cluster model, a nuclearity that was already suggested by the catalytic data. Whereas the dimeric clusters are very stable at 0 K, ab initio thermodynamic analysis calculations reveal the [Cu3(μ -O)3]2+ to be the most stable species at 700 K in O2 under dry conditions. Combined infrared spectroscopy and DFT calculations further show that the [Cu3(μ-O)3]2+ cluster is anchored to two framework Al atoms located at the pore mouth of the 8-MR side pockets (Fig. 2 shows the Cu cluster in the zeolite).

Figure 3: In situ XANES before activation and after activation in O2 at 450 °C for 1h.
After establishing the active Cu site structure, the formation and activation of the cluster in O2, as well as its interaction with CH4, was also studied using in situ XAS. The X-ray Absorption Near Edge Structure (XANES), as displayed in Fig. 3, clearly shows the predominant Cu2+ character of the activated Cu- MOR (weak pre-edge at 8977 eV and pronounced shoulder at 8987 eV). The decrease in white line intensity at 8997 eV upon activation is indicative for dehydration of the fresh sample leading to a change in coordination geometry from octahedral (hexa-aqua-complex) to tetrahedral in the [Cu3(μ -O)3]2+ cluster. EXAFS data of the sample in O2 as a function of temperature shows that from about 200 °C, Cu···Cu contributions are progressively formed, indicating the formation of the Cu-oxo clusters with increasing Cu nuclearity. Upon exposure to CH4 a strong XANES feature at 8983 eV is observed, indicative for the formation of Cu1+.
The corresponding EXAFS does not display significant changes, which indicates that interaction of methane with the Cu cluster reduces a fraction of the Cu sites without breaking up the trimeric cluster. Desorption of CH3OH can only be accomplished by steam treatment of the Cu-MOR catalysts, which leads to a substantial decrease in the Cu···Cu EXAFS contribution and an increase in the XANES white line intensity. It can thus be concluded that the steam treatment leads to the hydrolysis of the trimeric clusters. Reactivation in O2 at 500 °C however leads to a complete regeneration of the active species without loss of activity.
The observed reactivity of this trimeric Cu cluster in the mordenite zeolite is very similar to that of the trimeric Cu cluster in the particulate MMO (pMMO) enzyme2,3. It is shown in this study that the multiple Al framework atoms in the 8-membered ring side pocket of the MOR provide the conditions to stabilise the [Cu3(μ -O)3]2+ clusters. This stabilisation and the potential enhancement of the activity of the cluster is thought to be due to the similar steric constraints compared to the hydrophobic cavity formed by the pMMO subunits and therefore a truly biomimetic system has been synthesised and developed.
References:
- Grundner, S., Luo, W., Sanchez-Sanchez, M. & Lercher, J. A. Synthesis of single-site copper catalysts for methane partial oxidation. Chemical Communications 52, 2553-2556, doi:10.1039/c5cc08371k (2016).
- Bordeaux, M., Galarneau, A. & Drone, J. Catalytic, Mild, and Selective Oxyfunctionalization of Linear Alkanes: Current Challenges. Angewandte Chemie-International Edition 51, 10712-10723, doi:10.1002/ anie.201203280 (2012).
- Chan, S. I. et al. Efficient Oxidation of Methane to Methanol by Dioxygen Mediated by Tricopper Clusters. Angewandte Chemie-International Edition 52, 3731-3735, doi:10.1002/anie.201209846 (2013).
Funding acknowledgement:
The research was partly supported by the Inorganometallic Catalyst Design Center (ICDC), an Energy Frontier Research Center funded by the U.S. Department of Energy, Office of Basic Energy Sciences, Division of Chemical Sciences under Award DESC0012702. It was also partly supported by the EU NEXT-GTL (Innovative Catalytic Technologies & Materials for Next Gas to Liquid Processes) project. The XAS measurements were carried out at Diamond Light Source on Beamline B18 under Proposal SP8508. SurfSARA and NWO (The Netherlands Organisation for Scientific Research) are acknowledged for providing access to supercomputer resources.
Corresponding author:
Professor Moniek Tromp, University of Amsterdam, m.tromp@uva.nl.


 Diamond Annual Review 2016
Diamond Annual Review 2016

