 | ||
Structural investigation of novel anode materials for solid-oxide fuel cells |
Beamline I20 Scientific Highlight
The operation of a single fuel cell is depicted schematically in Fig. 11. A monocell is constituted by an anode, a cathode, and an electrolyte. As long as the fuel and oxidant are supplied to the anode and cathode respectively then the fuel cell continues to supply electrical power and produce reaction products such as water and carbon dioxide. The types of fuel cells under active development are the alkaline fuel cell (AFC), polymeric-electrolyte-membrane fuel cell (PEMFC), phosphoric-acid fuel cell (PAFC), molten-carbonate fuel cells (MCFGCs) and solid-oxide fuel cells (SOFCs), also in its reversible version (RSOFCs).
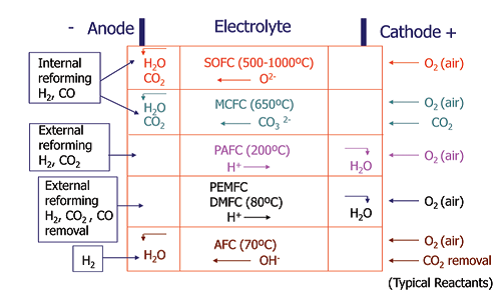
- Figure 1: Summary of fuel-cell types. The oxidation reaction takes place at the anode and involves the liberation of electrons (for example, O<sub>2</sub><sup>-</sup> + H<sub>2</sub> = H<sub>2</sub>O+2 e<sup>-</sup> or H<sub>2</sub> = 2H<sup>+</sup> + 2 e<sup>-</sup>). These electrons travel round the external circuit producing electrical energy by means of the external load, and arrive at the cathode to participate in the reduction reaction (for example, 1/2O<sub>2</sub> + 2 e<sup>-</sup> = O<sub>2</sub> - or ½ O<sub>2</sub>+ 2 H<sup>+</sup> + 2 e<sup>-</sup> = H<sub>2</sub>O).<sup>1</sup>
At present, the most common materials for SOFCs and RSOFCs are oxide ion conducting yttria-stabilised zirconia (YSZ) for the electrolyte, strontium-doped lanthanum manganite (LSM) for the cathode, nickel/YSZ for the anode, and doped lanthanum chromite or high-temperature metals for the interconnect. SOFC technology is efficient in generation of electricity from a variety of fuels for a wide range of power generation applications. However, the need to minimise cell resistivities implies the selection and processing of improved or of novel cell components. The fabrication and stability of the three-phase interfacial region within each electrode, ensuring that the electrolyte, reactants, and electronic species are in intimate contact, is of crucial importance to the electrochemical performance of a fuel cell. In this scenario, mixed conductors should be able to replace the current state of the art anodes for SOFCs. Both ionic and electronic defects are present in non-negligible concentrations in mixed conductors, and their mobility is so significant that the electrode reaction can proceed at any point on the electrode surface. The usual requirement of a triple phase contact, mentioned above, is replaced by a simple requirement of a two phase boundary region between electrode (mixed conductor) and gas phase.
Mixed conductors based on yttria-stabilised zirconia (YSZ) or yttriatetragonal zirconia polycrystalline (YTZP) are usually obtained if a significant amount of a mixed valence dopant cation is introduced in solid solution. In particular, titania-doped YSZ has been given much attention because of potential applications of mixed conduction under reducing conditions2. In addition, both YSZ (8 mol% Y2O3) and YTZP (3 mol% Y2O3) are oxygen ion conductors, but it was found that above 700 ºC the conductivity of YTZP is lower than in the cubic phase while it is a tougher material. These two combined characteristics, together with the electronic conduction associated to Ti ions make Ti-doped YSZ/Ti-doped YTZP composite materials a very attractive option for application in electrochemical devices, such as matrices in a SOFC anode.
To date, studies of the local environment of mobile ionic species have been only performed for tetragonal Ti-doped YTZP materials but not for cubic Ti-doped YSZ or Ti-doped YTZP/ Ti-doped YSZ composite materials. To better understand the role of titania on the ionic transport properties of zirconiabased solid solutions, the duplex materials (50 wt% Ti-YSZ/ 50 wt% Ti-YTZP) have been studied by using X-ray Absorption Spectroscopy (XAS). The Ti, Zr, and Y K-edge X-ray Absorption Near-Edge Structure (XANES) and Extended X-ray Absorption Fine Structure (EXAFS) spectra were collected at the scanning branch of the Versatile X-ray Absorption Spectroscopy beamline (I20-scanning) at Diamond, with the aim to identify the structural changes occurring upon the different processing as well as their relationship with the observed differences in their electrical performance.

Figure 2: Normalised XANES spectra at the Ti K-edge of the tetragonal 2Y12T2h sample and of the duplex materials, 10Ti8h and 15Ti2h (see text for details). The cubic phases extracted from the duplex samples have been added for comparison. The inset panel shows the comparison of the tetragonal and one of the extracted cubic phases with the spectrum of a pyrochlore Y2Ti2O7 reference sample.
Spectra have been recorded on duplex materials obtained by doping YSZ with 10 mol% of TiO2 and sintering in air for 8 h (10Ti8h) and doping YSZ with 15 mol% of TiO2 and sintering in air for 2 h (15Ti2h). In addition, a tetragonal (ZrO2)0.86(Y2O3)0.02(TiO2)0.12 sample sintered at 1500 ºC for 2 h in air (2Y12T2h) and a pyrochlore Y2Ti2O7 sample were also measured for reference purposes. The main goal of the XAS studies is to determine the local structure around the Ti in the cubic phase. However, this phase is only present in conjunction with the tetragonal phase in the duplex material. As XAS is an average technique, the coordination environment of the absorbing atom, Ti, Zr or Y, present in both phases will be probed simultaneously. Because the duplex materials contain a similar content of cubic and tetragonal phases, ~50% each, the XAS signals of the cubic phase have been obtained by subtracting the measured spectrum of the tetragonal reference sample with the appropriate 50% weighting.
XAS results, both XANES and EXAFS, show that Ti is sixfold coordinated in a disordered Ti-O arrangement in both the cubic and tetragonal phases of the duplex materials, departing from the eightfold coordination expected if a simple substitution at the Zr sites would take place. This is illustrated in the observed evolution of the strong pre-edge observed in the XANES spectra at energies around 4970 eV (Fig. 2). This peak should be very weak or absent in the case of a regular sixfold Ti-O arrangement, as occurring in the Y2Ti2O7 pyrochlore reference compound, and very sharp in fivefold or fourfold structures3 with an expected intensity much higher than the observed ones in the systems under studied. In contrast, the observed increase of the prepeak intensity in the duplex samples is in agreement with the occurrence of a distorted Ti-O arrangement either with an off-center displacement of the Ti atom within the octahedral TiO6 local bonding unit. These results indicate that starting from the strongly distorted sixfold coordination of Ti in the tetragonal phase, Ti in the cubic phase exhibits a tendency towards a non-centrosymmetric Ti-O polyhedron with interatomic distances close to those of pyrochlores or both rutile and anatase (1.95-1.96 Å). We have also observed that the Ti-O coordination polyhedron becomes more disordered as the Ti content increases. All in all, these results indicate that Ti ions would trap oxygen vacancies resulting in the overall decrease of the electrical conductivity in the titaniadoped materials with respect to the undoped materials, in agreement with the electrical conductivity behavior.
Source publication:
Colomer, M. T., Díaz-Moreno, S., Boada, R., Maczka, M. & Chaboy, J. Relationships between structural and electrical properties in mixed conductors duplex materials in the ZrO2-Y2O3-TiO2 ternary system. Physical Review B 89, doi:10.1103/PhysRevB.89.094101 (2014).
References:
1. Steele, B. C. H. & Heinzel, A. Materials for fuel-cell technologies. Nature 414, 345-352, doi:10.1038/35104620 (2001).
2. Colomer, M. T. & Maczka, M. Mixed conductivity, structural and microstructural characterization of titania-doped yttria tetragonal zirconia polycrystalline/titania-doped yttria stabilized zirconia composite anode matrices. Journal of Solid State Chemistry 184, 365-372, doi:10.1016/j. jssc.2010.12.006 (2011).
3. Sanjuan, M. L. et al. Raman and x-ray absorption spectroscopy study of the phase evolution induced by mechanical milling and thermal treatments in R2Ti2O7 pyrochlores. Physical Review B 84, doi:10.1103/ PhysRevB.84.104207 (2011).
Funding acknowledgements:
This work has been supported by Ministerio de Economía y Competitividad of Spain (MINECO) through the projects MAT2012- 31090 and MAT2011-27573-C04-04, and by the Aragón DGA NETOSHIMA grant. The authors thank Diamond Light Source for access to the Versatile X-ray Absorption Spectroscopy beamline, I20. We also wish to thank Mrs. C. Díaz- Dorado for the micrographs composition of this work. RB acknowledges support from the MINECO.
Corresponding author:
Dr Jesús Chaboy, Instituto de Ciencia de Materiales de Aragón, CSIC – University of Zaragoza, [email protected]


 A brighter light for science
A brighter light for science
