 | ||
Metal ions in bone cells – where and how? |
The hip joint is one of the largest joints in the human body and over the last decade it is estimated that more than half a million hips have been replaced in England and Wales as a result of osteoarthritis. Osteoarthritis is the most common joint disorder in adults, affecting over eight million people in the UK alone. Surgical replacement of the joint using artificial metal implants is one of the most effective ways to restore activity and reduce pain and disability in osteoarthritis sufferers. Hip implants rely on the normal functioning of bone cells to achieve fixation of the implant with the bone. However, small metal particles, cobalt (Co) and chromium (Cr), released from hip implants due to friction between the moving surfaces have been shown to be toxic to the surrounding bone cells. This causes the implant to loosen in the bone and often leads to patients requiring a second surgery to replace the failed implant. In this study, world-leading researchers from the University of Sheffield used the Microfocus Spectroscopy beamline (I18) at Diamond Light Source to measure the elemental distribution and characterise the chemical form of the metals in human bone cells. The findings revealed that the location of the metals that are released from implants is different between osteoblasts and osteoclasts, the two types of bone cells responsible for bone maintenance and repair. These results meet the overall aim of the research to better understand the cellular entry, trafficking, and processing of Co and Cr in bone cells, and identify possible tractable targets to mitigate their adverse effects after joint replacement.
Beamline I18 Scientific Highlight
Previous research has shown that Co and Cr ions at the levels observed in patients with Metal-on-metal joint replacements affect bone cell survival and function in-vitro1, and associate with systemically measureable effects on bone mass and bone turnover in patients2. However, it is not fully understood how metal ions enter bone cells or their speciation and distribution within the cell, information that is fundamental if we are to try and mitigate the effects of these ions. This study aimed to rectify this using Microfocus X-ray Fluorescence (μ-XRF) to measure the elemental distribution and Micro-X-ray Absorption Near-Edge Structure (μ-XANES) to characterise the chemical form of the metals in human bone cells.
We first treated human osteoblast-like cells for three days and human osteoclasts generated from peripheral blood1 at two time points, from day three to day 14 and from day 14 to day 21, thus representing the two distinct phases of developing and mature osteoclasts, with the relevant concentrations of metal ions3. Cells were then fixed and subject to μ-XRF and μ-XANES scanning using beamline I18 at Diamond. XRF elemental maps (Fig. 1) were produced for the different cell types and for the different metal ion treatments to localise the metals within the cell. When compared to the corresponding microscope images cobalt was present throughout the cell body in osteoblasts, but the signal was most intense in the area corresponding to the cell nucleus (Fig. 1). Within osteoclasts localisation of cobalt signals were to discrete areas within both developing (D-Oc) and mature cells (M-Oc), corresponding to the basolateral membrane in phase contrast images (Fig. 1). We next generated K-edge μ-XANES spectra from the intracellular sites with the highest cobalt concentrations and compared them to standards of cobalt at different oxidation states (Co-metal, CoO, Co2O3 and Co(II)acetate, Fig 1). Osteoblasts and mature osteoclasts showed the presence of cobalt in the +2 oxidation state corresponding to the Co(II)acetate spectra, indicating that no intracellular redox change occurs following entry of Co2+ ions into these cell types. Whilst developing osteoclasts demonstrated some cellular entry of Co2+, the concentration was insufficient to generate μ-XANES spectra, suggesting that uptake of cobalt is less for developing osteoclasts relative to active, mature osteoclasts. Similar XRF maps and μ-XANES spectra were produced for cells treated with Cr3+ and Cr6+, which revealed different intracellular localisation of chromium in osteoblasts compared to osteoclasts with Cr3+ treatments, whilst a similar diffuse distribution was observed in both cell types with Cr6+ treatments (Fig 1). No intracellular redox change was observed with Cr3+ treatments, but Cr6+ was observed to undergo an intracellular reduction to Cr3+ based on their μ-XANES spectra (Fig 1). The above observations are consistent with known interactions of these metal ions with DNA and nuclear proteins associated with DNA repair, which could account for their effect on bone cell viability. In addition, the data is consistent with the ability of the different cell types to undergo the processes of endo- and exo-cytosis and the expression of functional secretory domains4.
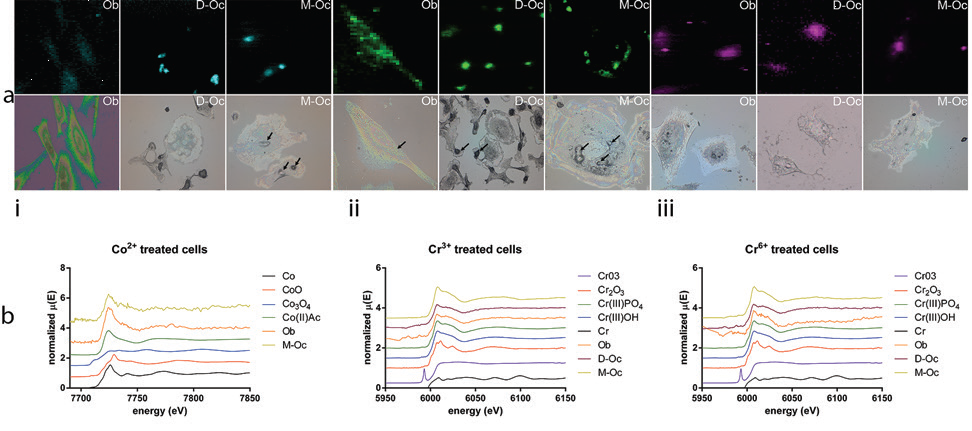
Figure 1: Distribution and speciation of metal ions in bone cells. (a) μ-XRF maps of metal ion treated bone cells (top-panels) with corresponding microscope images (bottom panels); (b) μ-XANES spectra for treated cells compared to standards of different oxidation states (Co-metal, CoO, Co2O3 and Co(II)acetate). i) Co2+ ii) Cr3+ and iii) Cr6+; Ob=osteoblasts, D-Oc=developing osteoclasts, M-Oc=mature osteoclasts.
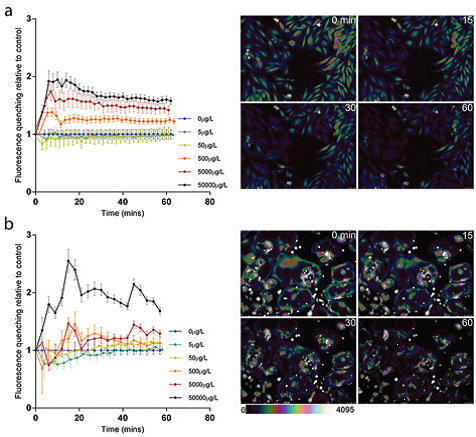
- Figure 2: Calcein-AM quenching by Co<sup>2+</sup> as a measure of cellular entry (a) Time-course data showing mean (95%CI) Calcein-AM quenching at differing concentrations of Co<sup>2+</sup> with representative fluorescence images and colour bar underneath for osteoblasts and (b) osteoclasts. Graphical data are plotted as the amount of fluorescence quenching relative to control, representing cellular entry of Co<sup>2+</sup> over time. Reproduced from Shah et al<sup>3</sup>.
This new information on the localisation of metal ions in bone cells was fundamental in generating the hypothesis that entry of Co2+ into osteoblasts is mediated by plasma membrane channels and transporters, as is the case with other divalent ions5. This led to testing the effect of blocking the plasma membrane channel P2X7R and the proton-coupled divalent metal transporter DMT-1, known to play a role in divalent ion entry in cells. Using Calcein-AM quenching by Co2+ as a measure of cellular entry (Fig.2), we were able to demonstrate that osteoblasts treated with Co2+ had a dose-dependent increase in cellular entry of Co2+ (Fig.3) and that treatment with the P2X7R antagonist A740003 reduced this. However, treatment of osteoblasts with the DMT-1 antagonist did not reduce cellular entry of Co2+ (Fig.3). Therefore it is concluded that the P2X7R contributes cellular entry of cobalt ions in osteoblasts, whilst the proton-coupled divalent metal transporter DMT-1, although expressed in human osteoblasts, does not contribute significantly to Co2+ transport in human osteoblasts. Addition of the P2X7R or DMT-1 antagonists did not affect cellular entry of Co2+ into osteoclasts at any concentration, consistent with a different intracellular localisation as stated above, and a different mechanism of metal entry.
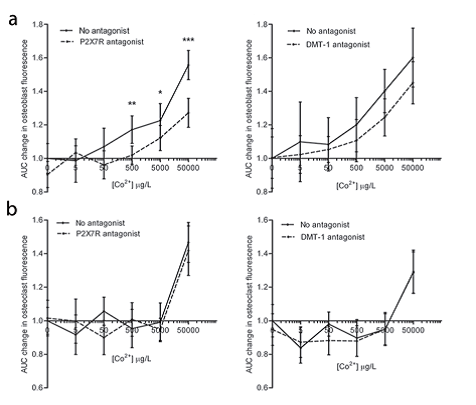
- Figure 3: Inhibition of Co<sup>2+</sup> entry by P2X7R antagonist. (a) Mean (95%CI) change (relative to control) in area under curve for quenching of Calcein-AM by Co<sup>2+</sup> in response to P2X7R antagonist (A740003) in bone cells; (b) Mean change in AUC in response to DMT-1 antagonist (NSC306711) in osteoblasts and osteoclasts. Analysis is antagonist versus no antagonist at each metal exposure by unpaired t-test; *P<0.05, **P<0.01. Reproduced from Shah et al<sup>3</sup>.
In conclusion, the intracellular distribution of metal ions highlights a celland metal-specific mode of cellular entry, whilst speciation confirms redox stability of Co2+ and Cr3+, and reduction of Cr6+ to Cr3+. Finally, the data suggests a role of P2X7R in cellular entry of Co2+ in osteoblasts, but not osteoclasts, identifying it as an investigative candidate for cell-specific targeting to modulate the osteoblast response at the bone-implant interface in presence of Co2+ tribocorrosion products.
Source publication:
Shah, K. M., Quinn, P. D., Gartland, A. & Wilkinson, J. M. Understanding the Tissue Effects of Tribo-Corrosion: Uptake, Distribution, and Speciation of Cobalt and Chromium in Human Bone Cells. Journal of Orthopaedic Research 33, 114-121, doi:10.1002/jor.22729 (2015).
References:
1. Andrews, R. E., Shah, K. M., Wilkinson, J. M. & Gartland, A. Effects of cobalt and chromium ions at clinically equivalent concentrations after metal-onmetal hip replacement on human osteoblasts and osteoclasts: Implications for skeletal health. Bone 49, 717-723, doi:10.1016/j.bone.2011.06.007 (2011).
2. Prentice, J. R. et al. Metal-on-Metal Hip Prostheses and Systemic Health: A Cross-Sectional Association Study 8 Years after Implantation. Plos One 8, doi:10.1371/journal.pone.0066186 (2013).
3. Nesbitt, S. A. & Horton, M. A. Trafficking of matrix collagens through bone-resorbing osteoclasts. Science 276, 266-269, doi:10.1126/ science.276.5310.266 (1997).
4. Sheridan, E. J. et al. Synchrotron X-ray fluorescence studies of a brominelabelled cyclic RGD peptide interacting with individual tumor cells. Journal of SynchrotronRadiation 20, 226-233, doi:10.1107/s0909049513001647 (2013).
Funding acknowledgement:
We would like to thank National Institute for Health Research for funding this work via a NIHR Biomedical Research Fellowship (BRF-2011- 013 and BRF-2011-020) to K.Shah.
Corresponding author:
Dr Alison Gartland, University of Sheffield, a.gartland@sheffield.ac.uk


 A brighter light for science
A brighter light for science
