Beamline B23 Scientific Highlight
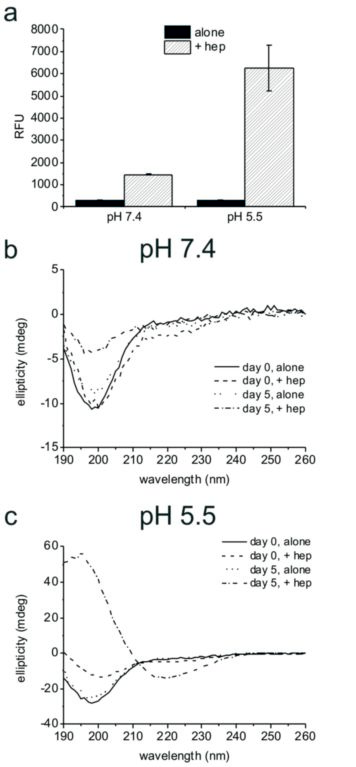
- Figure 1: Analysis of PLB(1-23) aggregation. (a) ThT fluorescence (y axis shows relative fluorescence units) for 2 mg/ml PLB(1-23) incubated at 37 °C with shaking for five days in the absence and presence of 2mg/ml heparin at pH 7.4 or pH 5.5, mean and standard error of the mean are shown for n=3. SRCD spectra of 2 mg/ml PLB(1-23) at pH 7.4 (b) and pH 5.5 (c) freshly prepared (day 0) and pre-incubated at 37 °C for five days, in the presence and absence of 2 mg/ml heparin as shown. Spectra are an average of 4 scans following dilution to 0.5 mg/ ml in buffer and are shown following subtraction of buffer and heparin control spectra.
Previous research has shown that the anticoagulant heparin can induce amyloid formation in a number of peptides that do not aggregate in the absence of heparin. The chosen peptide for this study, the 23-residue peptide PLB(1-23), has been shown to bind heparin-derived polymers of saccharides. Peptide aggregation was studied using several methods with samples incubated under a series of conditions. Using the Circular Dichroism beamline (B23) at Diamond Light Source, the change in protein folding in the peptide structure and its associated amyloid aggregation could be observed.
Amyloid fibril formation was assessed by using the fluorescent dye thioflavin T, which shows increased fluorescence in the presence of the crossbeta structure associated with amyloids. An increase in fluorescence was seen solely for the samples containing heparin, suggesting that PLB(1-23) remains unfolded in solution in the absence of a biological trigger, but that fibrils assemble when heparin is present. This is most pronounced at pH 5.5. The results suggest heparin could act as a template to enhance aggregation by aligning the peptide molecules in the correct orientation to cause growth of amyloid fibrils.
Amyloid deposits in vivo are complex mixtures composed of protein fibrils and non-fibrillar components including polysaccharides of the glycosaminoglycan (GAG) class. It is widely documented that GAGs influence the initiation and progress of self-assembly by several disease-associated amyloidogenic proteins and peptides in vitro. Heparin has also been shown to induce amyloid fibril formation by a range of hormone peptides1. This study investigated the ability of the GAG heparin to serve as a cofactor to induce amyloid-like fibril formation in a peptide predicted to have a low propensity to aggregate and not associated with amyloid disorders. The 23-residue peptide PLB(1-23) was chosen, which corresponds to the natively N-terminally acetylated cytoplasmic domain of the phospholamban transmembrane protein that is expressed predominantly in the sarcoplasmic reticulum of cardiac myocytes2. PLB(1-23) has previously been shown to bind heparinderived oligosaccharides with micromolar affinity3.
Several methods were employed to investigate the effect of heparin on the propensity of PLB(1-23) to aggregate. To initiate aggregation, PLB(1-23) was dissolved to 2 mg/ml (0.72 mM peptide) in D-mannitol buffer, pH 5.5 or 20 mM sodium phosphate, pH 7.4, alone or with 2 mg/ml (approximately 0.4 mM) low molecular weight (5 kDa) heparin. Samples were incubated at 37 °C with shaking at 200 rpm for five days.
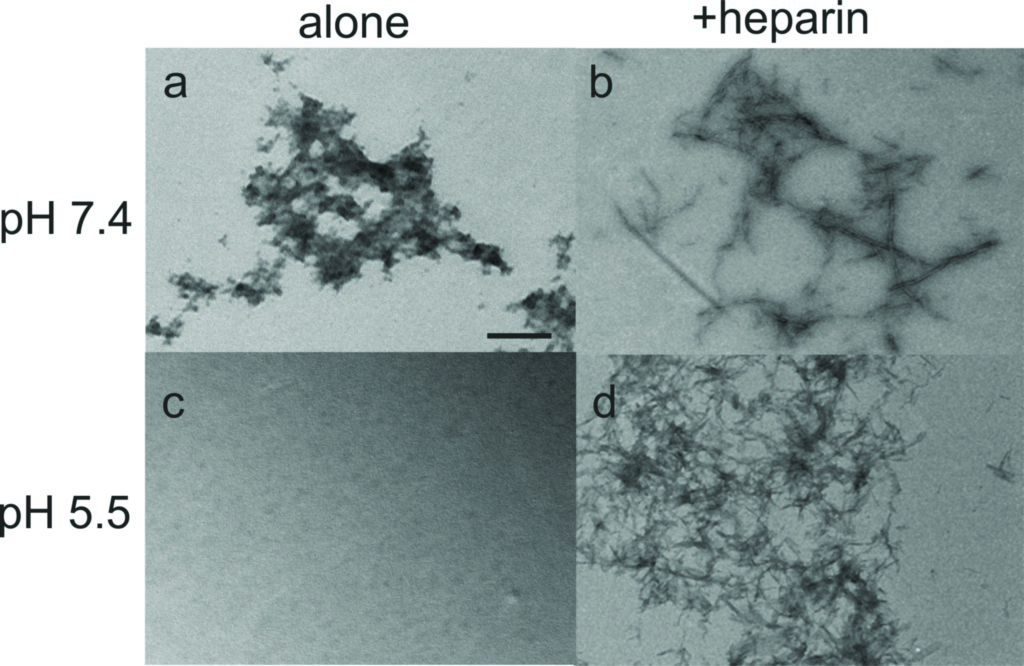
The SRCD spectra for PLB(1-23) alone after five days were consistent with the peptide remaining as an irregular structure at pH 7.4 and pH 5.5 (Fig. 1b,c, dotted lines). After five days in the presence of heparin at pH 7.4 an overall loss of signal intensity was seen, which was attributed to a reduced concentration of peptide in solution owing to precipitation (Fig. 1b, dot-dash line). Secondary structure fitting estimated that approximately 54% of the peptide was β-sheet or turn at day five. SRCD measurements for the peptide incubated in the presence of heparin at pH 5.5 for five days indicated that the peptide adopted virtually 100% β-sheet structure with negative ellipticity between 210–220 nm and a positive peak between 195–200 nm (Fig. 1c, dot-dash line), consistent with characteristic amyloid fibril structure4. ThT measurements shown in Fig 1a, reflect the β-sheet/turn content of the peptide observed by SRCD at day five. Hence the combination of a mildly acidic pH and the presence of heparin is sufficient for complete aggregation of PLB(1-23) into amyloid-like fibrils.
Source publication:
Madine, J., Davies, H. A., Hughes, E. & Middleton, D. A. Heparin Promotes the Rapid Fibrillization of a Peptide with Low Intrinsic Amyloidogenicity. Biochemistry 52, 8984-8992, doi:10.1021/bi401231u (2013).
References:
1. Maji, S. K. et al. Functional Amyloids As Natural Storage of Peptide Hormones in Pituitary Secretory Granules. Science 325, 328-332, doi:10.1126/science.1173155 (2009).
2. Fujii, J. et al. Complete complementary dna-derived amino-acid-sequence of canine cardiac phospholamban. Journal of Clinical Investigation 79, 301- 304, doi:10.1172/jci112799 (1987).
3. Hughes, E., Edwards, R. & Middleton, D. A. Heparin-derived oligosaccharides interact with the phospholamban cytoplasmic domain and stimulate SERCA function. Biochemical and Biophysical Research Communications 401, 370-375, doi:10.1016/j.bbrc.2010.09.056 (2010).
4. Nelson, R. et al. Structure of the cross-beta spine of amyloid-like fibrils. Nature 435, 773-778, doi:10.1038/nature03680 (2005).
Funding acknowledgements:
This work was funded by the Wellcome Trust (grant number 088563/B/09/Z). We are grateful to Diamond Light Source Ltd. for beamtime allocation (beamline B23), and thank Giuliano Siligardi and team for their guidance.
Corresponding author:
Dr Jillian Madine, University of Liverpool, j.madine@liv.ac.uk


 A brighter light for science
A brighter light for science

