 | ||
Three-dimensional micron-scale imaging of electrodeposited lithium microstructures |
Data gathered at the Diamond Manchester Imaging Branchline (I13-2) have been used to gain a better understanding of lithium battery failure. Lithium-ion batteries provide high power and energy densities, with cells containing pure lithium electrodes theoretically offfering the highest energy storage density by weight and volume. However, lithium metal is unsuitable for commercial use in batteries because it is considered inherently unsafe. Repeated charging and discharging results in electrodeposition of lithium into tree-like dendritic deposits, termed ‘lithium moss’. These deposits continually grow and can short-circuit the battery, leading to battery failure and potentially to fires or explosions.
Beamline I13 Scientific Highlight
Imaging lithium eletrodeposits in three dimensions is very difficult due to the low density of lithium, which renders them almost transparent to X-rays. The I13-2 branchline, which is part of the X-ray Imaging and Coherence beamline (I13), is a ten-year collaboration between the University of Manchester and Diamond Light Source, and offers intense, monochromatic X-rays, enabling high-resolution images to be obtained over short timescales to provide intricate details about the microstructure of lithium moss. These results represent the first characterisation of electrodeposited lithium metal in the environment in which they grew, as opposed to data collected using electron microscopy techniques which typically require removal of the deposits before analysis. The images have a sufficiently high signal-to-noise ratio such that they yield information on regions of differing electron density Materials Village Beamline I13 in the material. This indicates the formation of lithium compunds in addition to elemental lithium. The micron-resolution images pave the way for highly detailed analyses of lithium moss, with a view to improving safety and battery life for future applications.

Figure 1: The electrochemical cell mounted on the beamline in front of the detector setup.
Lithium dendrites or ‘moss’ can form within batteries which use metallic lithium an electrode. These metallic microscale networks form electrochemically during the charging process, but are not symmetrically removed when the current direction is reversed. This irreversible accumulation of lithium metal dendrites prevents the commercial adoption of lithium metal anodes in rechargeable batteries despite being the ideal material in terms of energy density. Whilst studies have been made of electrochemically deposited lithium microstructures using two-dimensional microscopy1, there has been relatively little three-dimensional analysis2. At I13-2 we used X-ray phase contrast microtomography to obtain 3D structural characterisation of dendritic lithium moss formed under different charging conditions, providing new insights into the dendritic growth process
Lithium (Li) presents unique challenges for tomographic imaging: its low bulk density causes limited absorption contrast, and it is unstable when exposed to air, rapidly forming hydroxide, nitride, or carbonate compounds. By creating a sealed lithium-lithium cell within a 1 mm diameter kapton capillary tube it was possible to electrodeposit lithium dendrites within a protective environment whilst allowing X-ray images to be obtained during a 180° rotation, as shown with the cell on I13-2 (Fig. 1). A 2 mm gap between electrodes was filled with 1M LiPF6 in 1:1 by volume Ethylene Carbonate:Dimethyl Carbonate, and copper wires at either end inserted into the lithium electrodes allowed a current to be passed through the cell, transferring Li ions between the electrodes mimicking the electrochemistry in a lithium metal battery. We used a monochromatic 19 keV beam, providing strong in-line phase contrast imaging. The PCO 4000 camera provided a 0.45 μm effective pixel size, achieving ~1 μm spatial resolution. To create tomography volumes we first ran a phase backpropagation filter to reduce the interference effects3 on a sequence of 1800 images, and then used a filtered back-projection 3D reconstruction4.
Figures 2 and 3 provide a comparison of the microstructures formed under constant current (Fig. 2) and cycled current (Fig. 3). The full 3D complexity of these different Li microstructures, and their interaction with the bulk lithium electrode, is revealed in the 3D rendering: under the constant current the mossy, non-faceted structures have the same X-ray attenuation as the metallic Li electrode, demonstrating that Li metal moss is formed. When the cell is cycled galvanostatically, more highly attenuating structures are formed, seen as brighter tree-like formations. The greater X-ray attenuation relates to an increased density due to the incorporation of higher atomic number elements in this structure, potentially formed via decomposition of components in the electrolyte.


Figure 2: Lithium moss formed under constant current (left) as a section through the reconstructed volume, and (right) a 3D surface rendering.

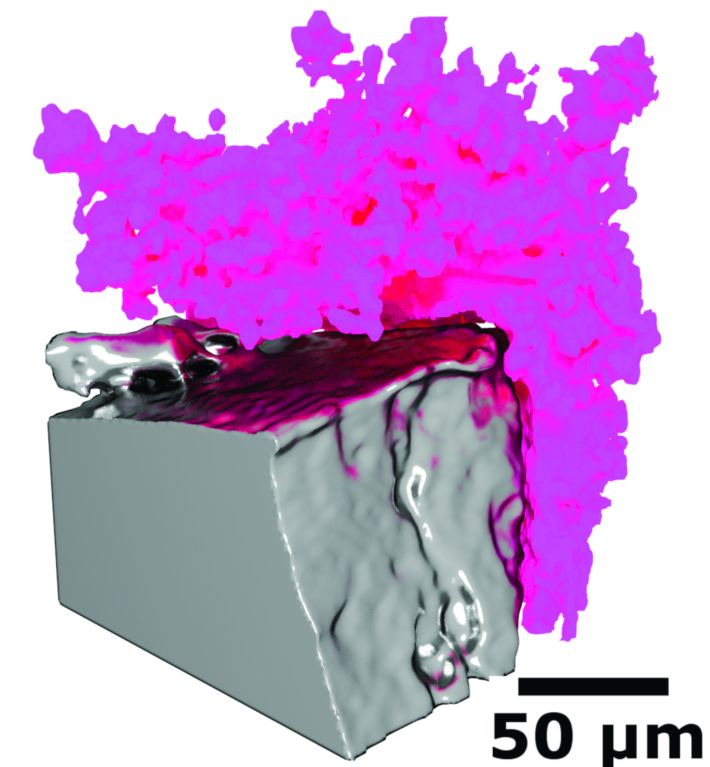
Figure 3: Lithium moss formed under cycled current (left) as a section through the reconstructed volume, and (right) a 3D surface rendering.
Source publication:
Eastwood, D. S., Bayley, P. M., Chang, H. J., Taiwo, O. O., Vila-Comamala, J., Brett, D. J. L., Rau, C., Withers, P. J., Shearing, P. R., Grey, C. P. & Lee, P. D. Three-dimensional characterization of electrodeposited lithium microstructures using synchrotron X-ray phase contrast imaging. Chemical Communications 51, 266-268, doi:10.1039/c4cc03187c (2015).
References:
1. Harris, S. J., Timmons, A., Baker, D. R. & Monroe, C. Direct in situ measurements of Li transport in Li-ion battery negative electrodes. Chemical Physics Letters 485, 265-274, doi:10.1016/j.cplett.2009.12.033 (2010).
2. Harry, K. J., Hallinan, D. T., Parkinson, D. Y., MacDowell, A. A. & Balsara, N. P. Detection of subsurface structures underneath dendrites formed on cycled lithium metal electrodes. Nature Materials 13, 69-73, doi:10.1038/ nmat3793 (2014).
3. Paganin, D., Mayo, S. C., Gureyev, T. E., Miller, P. R. & Wilkins, S. W. Simultaneous phase and amplitude extraction from a single defocused image of a homogeneous object. Journal of Microscopy 206, 33-40, doi:10.1046/j.1365-2818.2002.01010.x (2002).
4. Titarenko, S., Titarenko, V., Kyrieleis, A. & Withers, P. J. A priori information in a regularized sinogram-based method for removing ring artefacts in tomography. Journal of Synchrotron Radiation 17, 540-549, doi:10.1107/ s0909049510010964 (2010).
Funding acknowledgements:
The authors thank Diamond Light Source for the award of beamtime (MT8665) and the I13 beamline staff. DSE and PDL are also affiliated with the Research Complex at Harwell. DSE was supported by the EPSRC grant Structural evolution across multiple time and length scales (EP/ I02249X/1). PMB acknowledges FP7 Marie Curie International Incoming Fellowship, and PRS acknowledges the Royal Academy of Engineering for financial support.
Corresponding author:
Dr David Eastwood, University of Manchester; Diamond Manchester Collaboration, david.eastwood@manchester.ac.uk


 A brighter light for science
A brighter light for science
